6 Abdomen and Pelvis
Approach
CT is the primary imaging study for evaluation of most patients with major trauma and acute abdominal pain. In multiorgan trauma, a chest radiograph, pelvis radiograph, and focused abdominal ultrasound are usually performed during initial clinical assessment to determine whether surgical exploration should precede further imaging. In the hemodynamically stable patient, CT is the next step, and several imaging strategies can be pursued depending on the mechanism of injury and clinical findings.
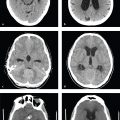
For trauma limited to the head, non-contrast head and cervical spine CT is performed, sometimes with the addition of cranial or neck CTA. Most patients with multiorgan trauma will require head, cervical spine, and abdominal CT. If there is clinical or radiographic evidence of thoracic injury, the chest should be imaged with the abdomen in a single acquisition. Extremity CT can be added for patients with complex orthopedic or vascular injuries.
Noncontrast CT of the head, face, and cervical spine are obtained first, usually in a single helical acquisition. If the chest is to be studied, images are obtained in the arterial phase of contrast enhancement, followed by a pause sufficiently long to image the abdomen and pelvis in the late arterial or early portal venous phase. Oral contrast is not routinely administered in the trauma setting, although oral and/or rectal contrast can be given to evaluate suspected penetrating injuries to the bowel. Delayed (excretory phase) images are valuable for detection of vascular extravasation and renal collecting system injuries.
For nontraumatic abdominal emergencies, ultrasound is used to investigate biliary colic, pelvic pain in women, scrotal pathology in men, suspected appendicitis or intussusception in children, and lower-extremity deep venous thrombosis. For most other acute conditions, CT is performed. Abdominal CT is obtained with intravenous contrast in the portal venous phase and oral contrast administered 45 to 90 minutes prior to the study. Imaging in the arterial phase can be performed to evaluate mesenteric ischemia, aortic dissection, or aneurysm, and can also be used to improve visualization of the pancreas in patients with upper abdominal pain.
Oral contrast is important for bowel visualization but may be omitted if clinical concern is limited to hepatic or pancreatic pathology, in which case water can be substituted immediately before scanning. Scans for hyperemergent conditions such as ruptured aneurysm, aortic dissection, or high-grade bowel obstruction with suspected ischemia should not be delayed for administration of oral contrast. Although it is controversial, some centers forgo the use of oral contrast material for abdominal CT in all emergency patients.
Patients who have had a prior life-threatening adverse reaction to any contrast agent, medication, or allergen should not receive intravenous contrast unless the clinical benefit of imaging with contrast outweighs the risks. Patients with prior anaphylactoid reactions can be premedicated with steroids and antihistamines, which reduce the risk of minor reactions and are presumed to reduce the risk of severe ones. Alternative imaging, such as MRI or ultrasound, should be considered in this group.
Patients with estimated glomerular filtration rate of 30–60 or serum creatinine 1.5–2.0 are at increased risk of contrast-induced renal injury and should receive no more than 75 mL intravenous contrast and 500–1,000 mL oral or intravenous hydration before and after the examination.
Patients with estimated glomerular filtration rate of < 30 or serum creatinine > 2.0 should not receive intravenous contrast unless an alternative study is not possible and the benefits and risks have been reviewed with the referring clinician. The decision to use intravenous contrast should be documented in the patient′s chart.
Intravenous contrast is not necessary for investigation of uncomplicated renal colic and evaluation of most osseous abnormalities.
Anatomic Checklists
Radiograph
Heart and lung bases
Gas pattern (stomach, small bowel, colon)
Soft tissues (liver, spleen, kidneys, psoas margins)
Calcifications (gallstones, renal calculi, calcified masses)
Bones
Computed Tomography
Heart
Lung bases
Diaphragm
Liver
Gallbladder
Biliary system
Kidneys
Adrenal glands
Pancreas
Spleen
Esophagus
Stomach
Duodenum
Small bowel
Colon
Appendix
Mesenteric and retroperitoneal lymph nodes
Blood vessels
Spine, pelvis, and musculature
Bladder
Uterus and ovaries in women
Prostate and seminal vesicles in men
Ultrasound
Liver (including portal and hepatic veins)
Gallbladder
Common bile duct
Kidneys
Pancreas
Spleen
Abdominal aorta
Bladder
Imaging and Anatomy
Computed Tomography Protocols
Trauma
Indications: Abdominal trauma.
Technique: 300–700 mA, 120 kV
Phase 1: arterial: bolus track to aorta 150 HU (symphysis to pubis)
Phase 2: venous: 90 seconds (symphysis to pubis)
Phase 3: delayed: 7 min (symphysis to pubis)—optional
Oral contrast: none
IV contrast: 1.5 mL/kg at 4 mL/sec, followed by 20 mL saline
Images: 4-mm axial with coronal and sagittal reformation
Approximate radiation dose: 700 mGy
Upper Abdominal Disease
Indications: Epigastric pain, pancreatic mass, jaundice, cholecystitis.
Technique: 300–700 mA, 120 kV
Phase 1: arterial: 40 seconds (upper abdomen only)
Phase 2: venous: 90 seconds (symphysis to pubis)
Oral contrast: water (1 liter)
IV contrast: 1.5 mL/kg at 4 mL/sec
Images: 4-mm axial with coronal and sagittal reformation
Approximate radiation dose: 700 mGy
Lower Abdominal Disease
Indications: Appendicitis, diverticulitis, pelvic pain, acute abdomen.
Technique: 300–700 mA, 120 kV
Phase 1: venous: 90 seconds (symphysis to pubis)
Oral contrast: Dilute diatrizoate meglumine (Gastrografin; 1 liter); water may be substituted for suspected pancreatitis
IV contrast: 1.5 mL/kg at 3 mL/sec
Images: 4-mm axial with coronal and sagittal reformation
Approximate radiation dose: 700 mGy
Mesenteric Ischemia
Indications: Suspected mesenteric ischemia.
Technique: 300–700 mA, 120 kV
Phase 1: arterial: bolus track to aorta 150 HU (symphysis to pubis)
Phase 2: venous: 90 seconds (symphysis to pubis)
Oral contrast: water (0.5 liters)
IV contrast: 1.5 mL/kg at 4 mL/sec, followed by 20 mL saline
Images: 4-mm axial with coronal and sagittal reformation
Approximate radiation dose: 700 mGy
Abdominal Aortic Aneurysm or Dissection
Indications: Suspected abdominal aortic aneurysm or dissection.
Technique: 300–700 mA, 120 kV
Phase 1: noncontrast (symphysis to pubis)
Phase 2: arterial: bolus track to aorta 150 HU (symphysis to pubis)
Oral contrast: water (0.5 liters)
IV contrast: 1.5 mL/kg at 4 mL/sec, followed by 20 mL saline
Images: 4-mm axial with coronal and sagittal reformation
Approximate radiation dose: 700 mGy (1,600 mGy when combined with chest CT)
Renal Stone
Indications: Renal colic.
Technique: 300–700 mA, 120 kV
Phase 1: noncontrast, prone position (symphysis to pubis)
Oral contrast: None
IV contrast: 1.5 mL/kg at 3 mL/sec
Images: 4-mm axial with coronal and sagittal reformation
Approximate radiation dose: 700 mGy
Ultrasound Protocols
FAST (Focused Assessment for Trauma)
Indications: Blunt and penetrating abdominal trauma.
Probe: Abdominal curvilinear (1–8 MHz)
Views:
– Subxiphoid (pericardium)
– Right upper quadrant (Morrison pouch)
– Left upper quadrant (perisplenic, left perirenal)
– Suprapubic (perivesical)
Right Upper Quadrant
Indications: Cholelithiasis, jaundice, upper abdominal pain.
Probe: Abdominal curvilinear (1–8 MHz)
Views:
– Gallbladder (longitudinal and transverse)
– Common bile duct/hepatic artery
– Main portal vein (color and spectral Doppler)
– Right kidney (longitudinal and transverse)
– Pancreas (midline transverse)
– Sonographic Murphy sign (presence or absence)
– If gallstones are present, image in supine and decubitus positions for mobility
Measurements:
– Gallbladder diameter: maximum AP and lateral (normal < 4 cm)
– Gall bladder wall thickness (normal < 3 mm)
– Common bile duct (normal < 7 mm)
Renal
Indications: Hydronephrosis, suspected renal mass, renal calculus.
Probe: Abdominal curvilinear (1–8 MHz)
Views:
– Kidneys (sagittal and transverse, color Doppler of renal hilum)
– Bladder (show urinary jets at ureteropelvic junction)
– Prostate (males)
Measurements:
– Renal long axis, AP, transverse
– Diameter of any cyst or mass
Scrotal
Indications: Pain, mass, hernia.
Probe: Linear probe (12 MHz or higher)
Views:
– Three transverse views of both testes on one image (upper, lower, mid)
– Three sagittal views of each testicle
– Epididymis (sagittal and transverse)
– Color and arterial flow Doppler both testes (sagittal and transverse)
– Inguinal region ± Valsalva for hernia
Measurements:
– Dimensions of each testis
Female Pelvic
Indications: Pain, mass, hernia.
Probes: Abdominal curvilinear (1–8 MHz), transvaginal
Views:
– Uterus (sagittal and transverse, transabdominal and transvaginal)
– Endometrium
– Ovaries with color and arterial flow Doppler
– Any adnexal masses
Measurements:
– Uterus (sagittal and transverse)
– Endometrium
– Ovaries
– Any mass or cyst > 1 cm
Obstetric First Trimester
Indications: Pain, bleeding.
Probes: Abdominal curvilinear (1–8 MHz), transvaginal
Views:
– Uterus (sagittal and transverse)
– Gestational sac/yolk sac/embryo
– Placenta (position)
– Ovaries with color and arterial flow Doppler
– Adnexa
Measurements:
– Record date of last menstrual period
– Uterus (sagittal and transverse)
– Cervical length
– Ovaries
– Any ovarian cysts > 1 cm
– Gestational sac
– Yolk sac
– Embryo crown–rump length
– Fetal heart rate by M-mode (avoid color Doppler)
– If no heart rate and fetal pole is > 5 mm, check with color Doppler
Lower Extremity Venous Doppler
Indications: Suspected deep venous thrombosis.
Probes: Linear probe (5–12 MHz)
Views:
– Sagittal with Doppler and augmentation
– Transverse with compression
– Common femoral vein
– Superficial femoral vein (proximal, mid, distal)
– Popliteal vein
– Posterior tibial and anterior tibial veins (color Doppler)
Anatomy
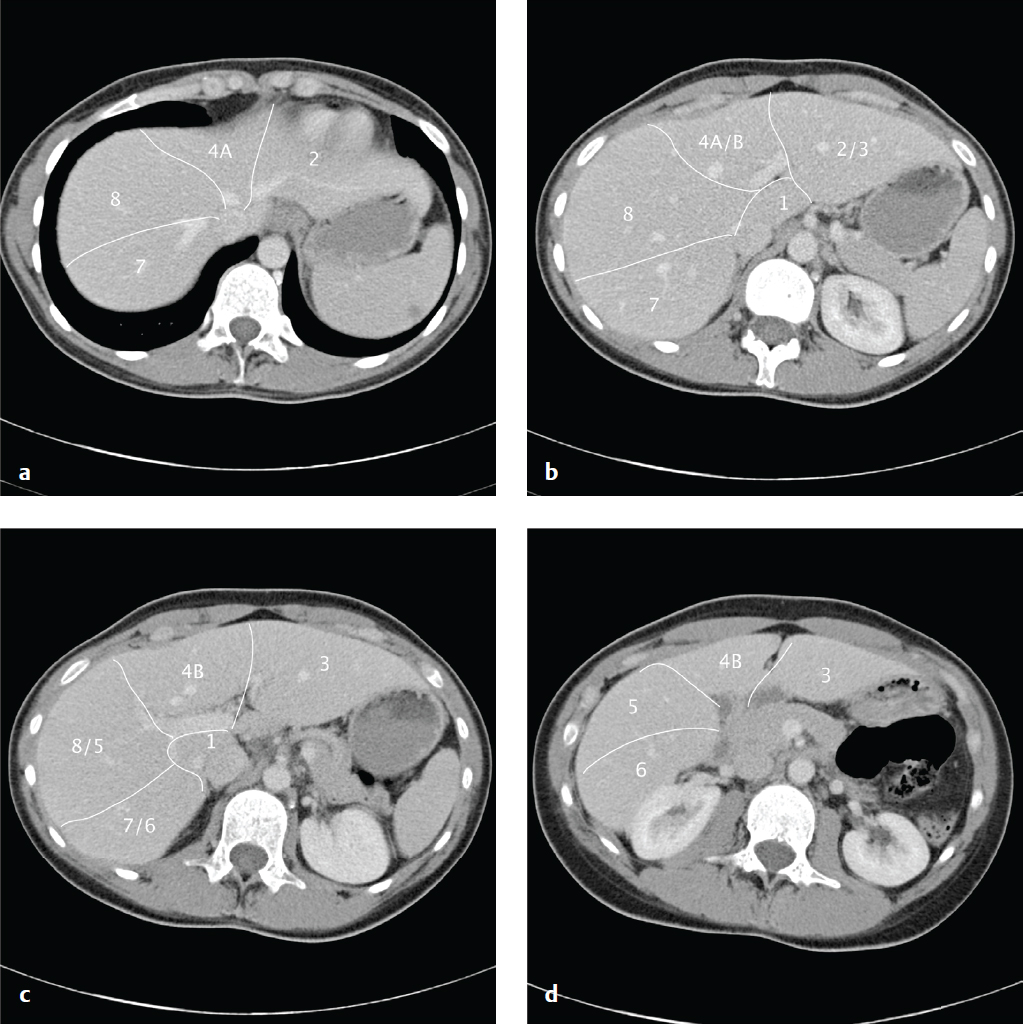
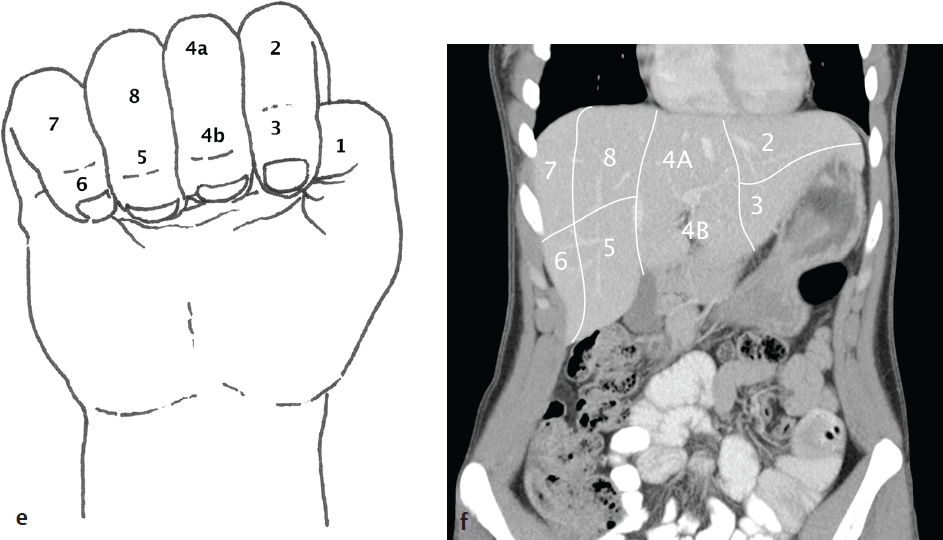
Hepatic Segmental and Subsegmental Anatomy
The liver is morphologically divided into a right lobe, left lobe, and caudate lobe. The right and left lobes can be divided into subsegments based on vascular supply and biliary drainage that permit partial hepatic resection for tumor ( Fig. 6.1 ).
Clinical Presentations and Differential Diagnosis
Clinical Presentations and Appropriate Initial Studies
Trauma
CT abdomen and pelvis with intravenous contrast.
Diaphragmatic rupture
Splenic, renal, or hepatic laceration
Bowel contusion/perforation
Hemoperitoneum
Retroperitoneal hematoma
Renal or hepatic arterial pseudoaneurysm
Renal artery dissection
Pelvic fracture/hematoma
Intra- or extraperitoneal bladder rupture
Spinal fracture
Upper Abdominal Pain
CT abdomen and pelvis with intravenous contrast (oral contrast material is usually not necessary, although water immediately prior to the examination improves gastric and duodenal distension). Ultrasound for biliary colic.
Cholelithiasis/cholecystitis
Gastritis
Gastric or duodenal ulcer
Pancreatitis
Biliary obstruction
Hepatic abscess
Thoracic pathology
Subphrenic abscess
Lower Abdominal Pain
CT abdomen and pelvis with intravenous and oral contrast.
Appendicitis
Diverticulitis
Small-bowel obstruction
Large-bowel obstruction
Inflammatory bowel disease (ulcerative colitis, Crohn disease)
Infectious enteritis/colitis
Epiploic appendagitis/omental infarct
Urolithiasis
Colon carcinoma
Abscess
Pelvic inflammatory disease
Ruptured or torsed ovarian cyst
Differential Diagnosis: CT
Pneumoperitoneum
Recent surgery
Peritoneal dialysis
Perforated gastric ulcer
Perforated duodenal ulcer
Perforated diverticulitis
Gasless Abdomen on Plain Radiograph
Proximal obstruction
Ascites
Vomiting
Normal variation
Stomach Mass
Malignant neoplasm (adenocarcinoma, lymphoma, gastrointestinal stromal tumor, metastasis)
Leiomyoma
Lipoma
Bezoar (intraluminal)
Hyperplastic, adenomatous, or hamartomatous polyp
Diffuse Gastric Wall Thickening
Gastritis
Pancreatitis
Crohn disease
Lymphoma
Diffuse Duodenal Wall Thickening or Mass
Duodenitis
Adenocarcinoma
Lymphoma
Metastasis
Crohn disease
Duodenal diverticulum
Hematoma
Enhancing Small-Bowel Wall
Shock bowel (avid mucosal enhancement)
Inflammatory bowel disease
Malignant neoplasm (adenocarcinoma, lymphoma, gastrointestinal stromal tumor, metastasis)
Radiation enteritis
Concentric Enhancement (Halo or Target Signs): Almost Always Nonmalignant
Inflammatory bowel disease
Infectious enteritis
Ischemic enteritis
Vasculitis
Angioedema
Radiation
Focal Small-Bowel Wall Thickening
Neoplasm (adenocarcinoma, lymphoma, gastrointestinal stromal tumor, metastasis)
Perforation
Crohn disease
Diverticulitis
Segmental Small-Bowel Wall Thickening
Crohn disease
Enteritis
Ischemia
Lymphoma
Hemorrhage
Diffuse Small-Bowel Wall Thickening
Enteritis
Ischemia
Hypoalbuminemia
Vasculitis
Terminal Ileal Wall Thickening/Inflammation
Inflammatory bowel disease
Tuberculosis
Bacterial infection
Lymphoma
Diffuse Colitis
Pseudomembranous colitis
Ulcerative colitis
Other infectious colitis
Right-Sided Colitis
Crohn disease
Neutropenic enterocolitis
Tuberculosis
Salmonella
Yersinia
Left-Sided Colitis
Ulcerative colitis (contiguous, distal)
Ischemic colitis (splenic flexure, sigmoid, tends to spare rectum)
Diverticulitis
Cecal Mass
Appendicitis
Cecal carcinoma
Appendiceal mucocele
Intestinal Pneumatosis
Ischemia
Obstruction
Toxic megacolon
Idiopathic (usually limited to colon)
Postendoscopy
Corticosteroids
Chemotherapy
“Misty” Mesentery
Pancreatitis
Cholecystitis
Diverticulitis
Congestive heart failure
Peritoneal carcinomatosis
Lymphoma
Sclerosing mesenteritis
Biliary Dilatation
Age-related
Postsurgical
Cholangiocarcinoma
Ampullary carcinoma
Gallbladder carcinoma
Pancreatic carcinoma
Hepatic metastases
Porta hepatis adenopathy
Common duct stone
Stricture
Mirizzi syndrome (stone in cystic duct)
Bile duct cyst
Gallbladder Wall Thickening (> 3 mm)
Cholecystitis
Hepatitis
Hypoalbuminemia
Cirrhosis
Congestive heart failure
Renal failure
Biliary System Gas
Sphincterotomy
Gallstone passage
Age-related
Postoperative
Biliary enteric fistula
Emphysematous cholecystitis (if air involves gallbladder wall)
Portal Venous Gas
Bowel infarction
Diverticulitis
Hepatomegaly
Hepatoma
Metastases
Lymphoma
Right-sided heart failure
Hepatitis
Hepatic Lesion: Low Attenuation on Nonenhanced CT
Hepatocellular carcinoma
Adenoma
Hemangioma
Focal nodular hyperplasia
Metastases
Cyst
Abscess
Hepatic Lesion: Arterial Phase Enhancing
Hepatoma
Hemangioma
Focal nodular hyperplasia
Adenoma
Metastasis
Hepatic Lesion: Portal Venous Phase Enhancing
Hepatoma
Venous collaterals
Hepatic Lesion: Delayed (Equilibrium) Phase Enhancing
Hemangioma
Cholangiocarcinoma
Treated metastases
Low-Attenuation Liver on Noncontrast CT
Fatty infiltration
Diffuse hepatoma or metastases
Budd-Chiari syndrome
Amyloid
Splenomegaly
Portal hypertension
Hemolytic anemias
Leukemia/lymphoma
Myelofibrosis
Malaria
Storage diseases
Sarcoidosis
Amyloidosis
Mononucleosis and other infections
Splenic Lesion
Lymphoma
Metastases
Hamartoma
Hemangioma
Sarcoid
Developmental or posttraumatic cyst
Abscess
Cystic Pancreatic Mass
Pseudocyst
Intraductal papillary mucinous neoplasm
Cystadenoma
Simple cyst
Metastasis
Abscess
Solid Pancreatic Mass
Adenocarcinoma
Focal pancreatitis
Metastasis
Islet cell tumor
Solid and papillary epithelial tumors
Solid Renal Mass
Renal cell carcinoma
Oncocytoma
Angiomyolipoma
Metastasis
Abscess
Infarct
Cystic Renal Mass
Simple cyst
Complex cyst
Renal cell carcinoma (mixed solid/cystic)
Abscess
Pelvic Mass
Ovarian cyst (simple, hemorrhagic, corpus luteum)
Ovarian neoplasm
Tubo-ovarian abscess
Uterine neoplasm
Rectal or sigmoid carcinoma
Prostate carcinoma
Bladder carcinoma
Differential Diagnosis: Ultrasound
Enlarged Ovary
Ovarian torsion
Ovarian neoplasm
Tubo-ovarian abscess
Hemorrhagic cyst
Hyperechoic Hepatic Mass
Hemangioma
Focal fatty infiltration
Metastasis
Hepatocellular carcinoma
Focal nodular hyperplasia
Hepatic adenoma
Hypoechoic Hepatic Mass
Hepatocellular carcinoma
Abscess
Focal fatty sparing
“Starry Sky” Liver
Acute hepatitis
Hepatocellular carcinoma
Diffuse metastatic disease
Hepatic congestion
Biliary or portal venous gas
Portal Vein Thrombosis
Cirrhosis
Hepatocellular carcinoma
Diffuse metastatic disease
Hypercoagulable state
Periportal infection
Common Bile Duct Dilatation
Choledocholithiasis
Chronic pancreatitis
Obstructing pancreatic or biliary malignancy
Cholangitis
Gallbladder Wall Thickening
Acute cholecystitis
Wall edema (hydration, congestive heart failure)
Hepatitis
HIV
Gallbladder carcinoma
Adenomyomatosis
Hyperechoic Renal Mass
Angiomyolipoma
Renal cell carcinoma
Complex cyst
Hypoechoic Renal Mass
Simple cyst
Lymphoma
Renal abscess
Renal cell carcinoma
Extratesticular Fluid Collection
Hydrocele
Varicocele
Hematocele
Pyocele
Adnexal Mass
Ectopic pregnancy (+ beta-hCG)
Corpus luteum cyst
Hemorrhagic cyst
Tubo-ovarian abscess
Endometrioma
Ovarian neoplasm
Enlarged Painless Testicle
Testicular neoplasm
Lymphoma/leukemia
Intratesticular cyst
Enlarged Painful Testicle
Orchitis/epididymitis
Torsion
Trauma (hematoma/fracture)
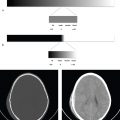
Diaphragm Rupture
Blunt impact to the abdomen can cause an abrupt elevation of intra-abdominal pressure and result in diaphragmatic rupture. Abdominal viscera can herniate through the defect, with acute or delayed bowel obstruction. Penetrating trauma to the back or upper abdomen can also result in diaphragm laceration. In both cases, the diagnosis can be missed or delayed, especially in patients who are placed on assisted ventilation immediately after resuscitation. Because positive intrathoracic pressure serves to prevent visceral herniation through any small diaphragmatic defect, the integrity of the diaphragm should be directly assessed, particularly in the ventilated patient. Remote diaphragmatic injury is a consideration in any patient with acute bowel obstruction and a past history of significant torso trauma.
Sagittal and coronal CT reformations best evaluate the diaphragm. Direct imaging signs of rupture or laceration include diaphragmatic discontinuity, waistlike constriction of herniated viscus (collar sign), and the dependent viscera sign, in which abdominal viscera appear to contact the posterior thoracic wall. Indirect findings include diaphragmatic thickening, injury on both sides of the diaphragm, or hemothorax without obvious thoracic injury, all of which indicate potential rupture. Penetrating injuries are less likely than blunt injuries to result in organ herniation, as the diaphragmatic defect is smaller (1 cm for penetrating injury compared with 5–6 cm in blunt trauma) ( Fig. 6.2 ).
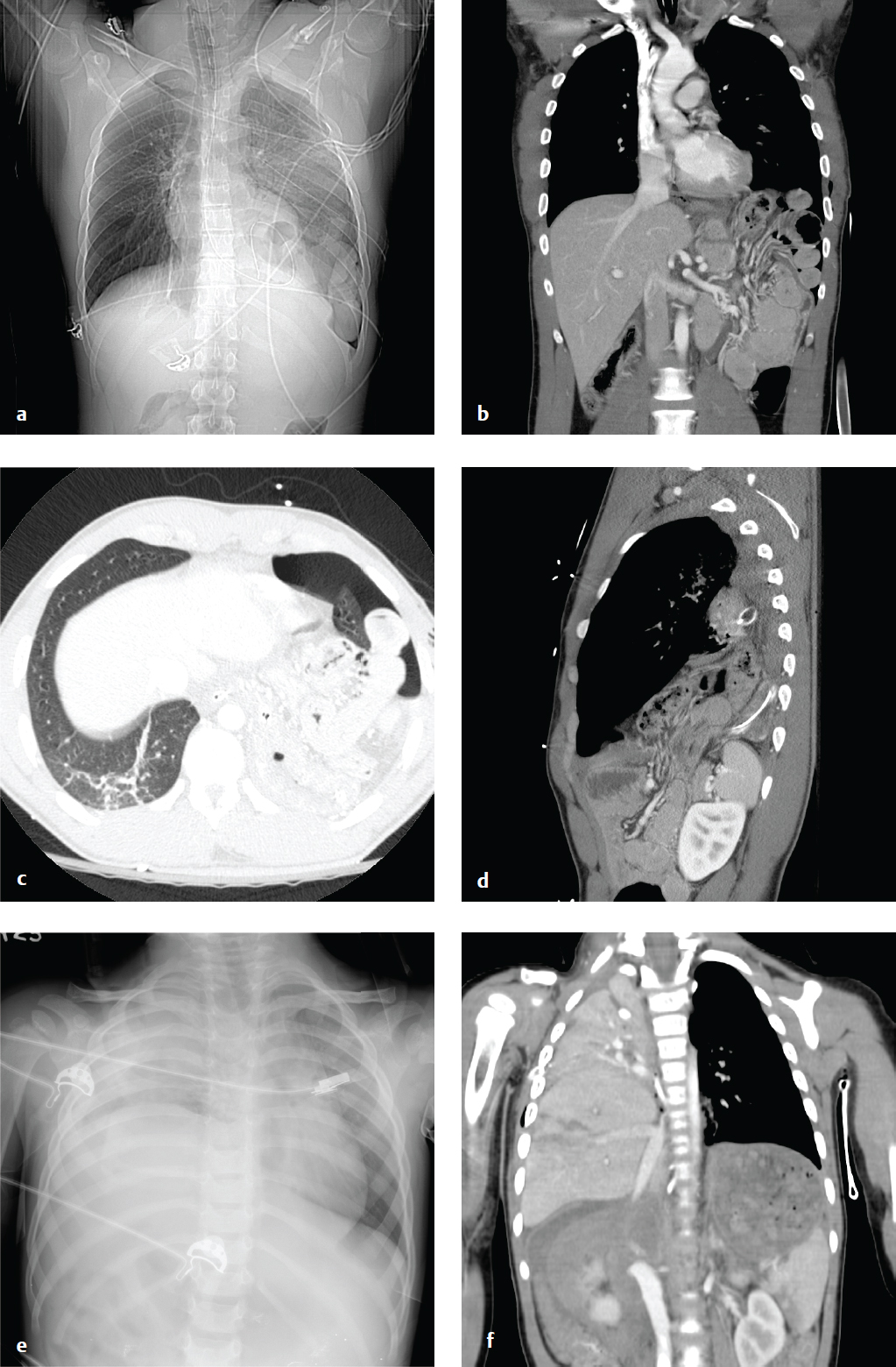
Splenic Injury
Splenic hematoma, laceration, devascularization, or frank rupture are potential consequences of blunt or penetrating injuries to the left torso. In patients with mononucleosis, the spleen is often enlarged and predisposed to rupture in minor abdominal trauma. Patients may present following trauma with diffuse abdominal pain or left upper quadrant tenderness. Associated left shoulder pain is referred to as the Kehr sign. Leukocytosis and hyperamylasemia may also be seen. Hemodynamic stability, rather than grade of injury, directs initial management and surgical decision making.
During initial evaluation and resuscitation, FAST (Focused Assessment with Sonography in Trauma) quickly identifies any intraperitoneal hemorrhage or obvious visceral injury. Contrast-enhanced CT scan is the most accurate test for evaluating the spleen and should be obtained in the hemodynamically stable patient.
On CT, lacerations appear as nonenhancing linear or jagged lesions, often at the splenic periphery. They are associated with pericapsular or subcapsular hematomas, a crescent-shaped soft tissue–attenuation fluid collection. This finding permits differentiation from splenic clefts, a normal variant. Splenic hematomas appear as low-attenuation intrasplenic masses. Acute contrast extravasation, particularly if increased on 5-minute delayed images, indicates active hemorrhage. Splenic artery pseudoaneurysms are contained vascular injuries that appear as small round or oval lesions with attenuation identical to that of other arteries on postcontrast studies. Extravasation and pseudoaneurysm are indications for emergent visceral arteriography.
Most classification systems grade splenic injury based on the size and location of subcapsular or parenchymal hematoma, length and depth of any laceration, and the presence or absence of active bleeding. Injuries that involve the vascular hilum may require embolization or splenectomy to control hemorrhage. Absent life-threatening hemorrhage, trauma surgeons increasingly prefer nonoperative management for splenic salvage. The American Association for the Surgery of Trauma (AAST) grading for splenic injury can be seen in Table 6.1 .
Depending on hemodynamic stability, most grade I–III injuries, as well as some of higher grade, can be managed nonoperatively. Grade IV and V injuries may require either arteriography/embolization or splenectomy ( Fig. 6.3 ).
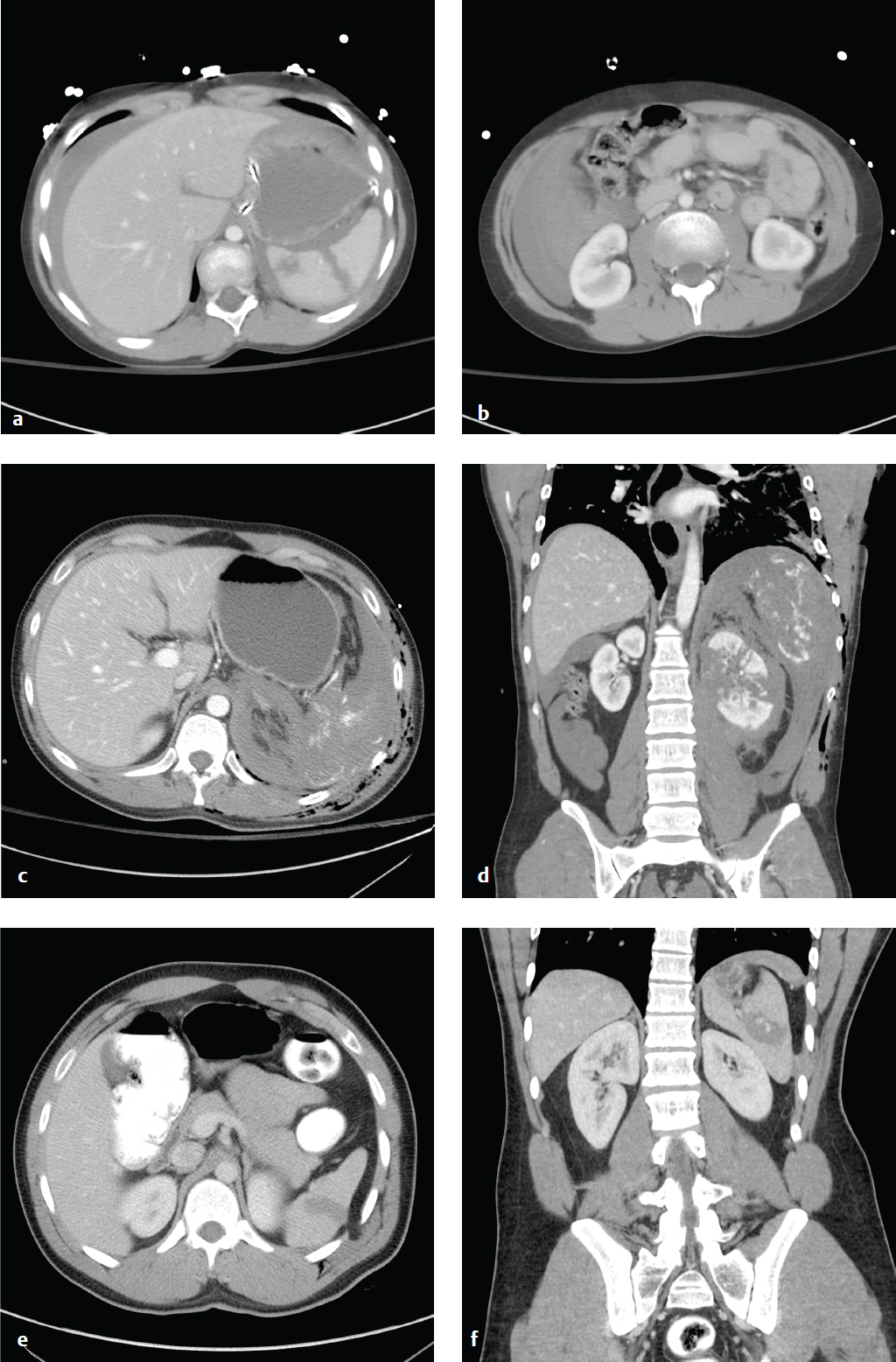
Hepatic Injury
The liver is the second most frequently injured organ in the abdomen, and hepatic injury is the most common cause of death in abdominal trauma. Blunt injuries include subcapsular or intraparenchymal hematoma, contusion, capsular disruption, laceration, and biliary disruption. Penetrating injuries from stab or gunshot wounds are usually managed nonoperatively or by angioembolization.
Contrast-enhanced CT localizes and characterizes parenchymal injury and detects other associated visceral injuries. As in the case of splenic injury, angiography and embolization are indicated for active extravasation or hepatic artery pseudoaneurysm. Similarly, the decision to operate or observe is dictated by the patient′s hemodynamic stability, the presence of active extravasation, or a decreasing hematocrit. Nonoperative management is generally preferred. High-grade injuries (IV–VI) should, if managed nonoperatively, be reimaged in 7 to 10 days, or sooner if there is a change in the patient′s clinical status. AAST grading for hepatic injury can be seen in Table 6.2 ( Fig. 6.4 ).
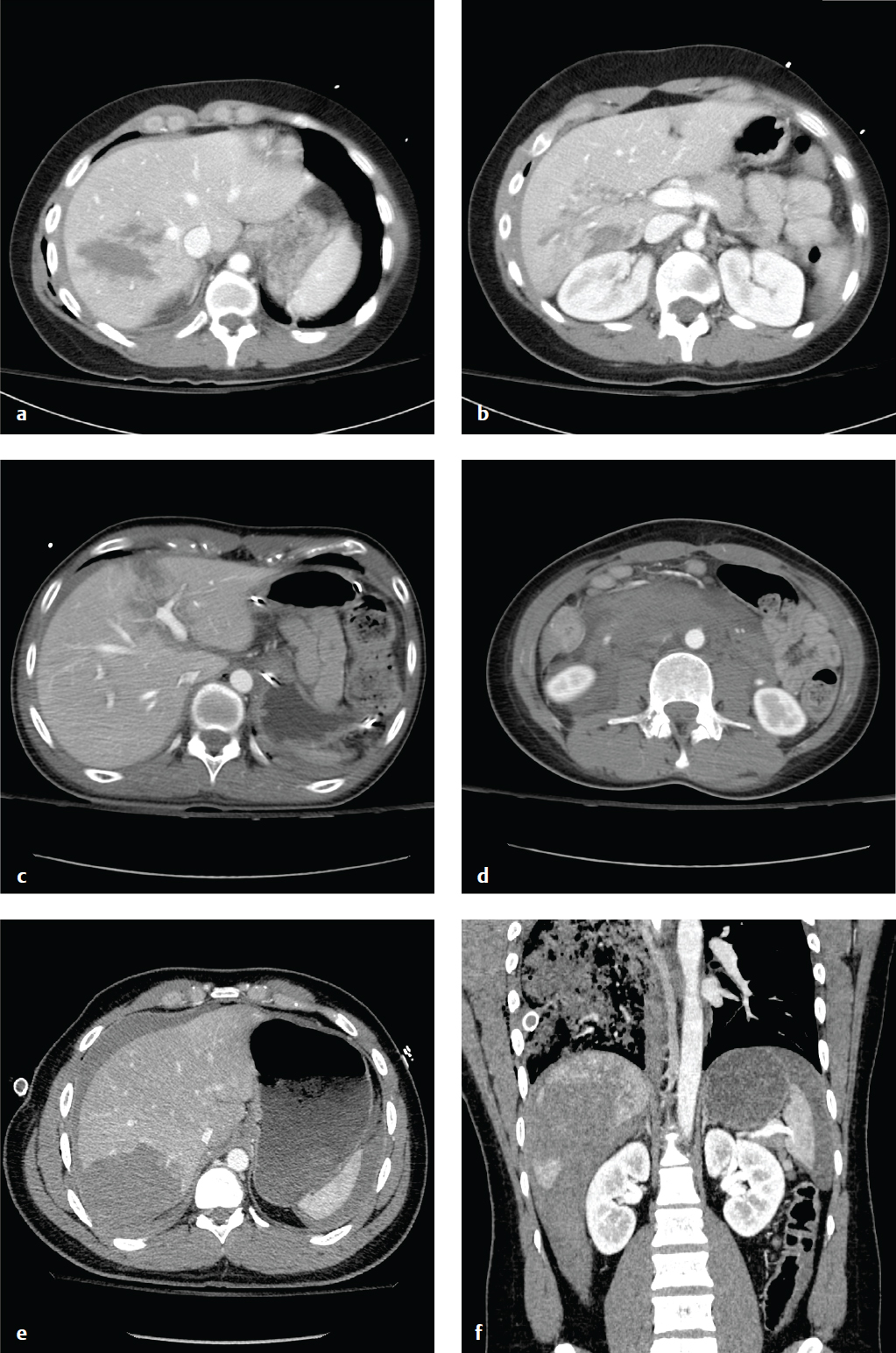
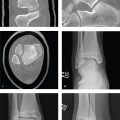
Renal Injury
Most renal injuries are due to blunt trauma, and concomitant visceral or skeletal injuries are often present. CT identifies subcapsular and parenchymal hematomas (contusion), renal lacerations, arterial extravasation, pseudoaneurysms, and collecting system injury. If a renal abnormality is found on initial workstation review, delayed images through the kidneys and collecting system should be obtained to detect arterial or urine extravasation.
Renal contusions are poorly defined, round, or ovoid low-attenuation areas and should be distinguished from renal infarcts, which are sharply delineated, usually peripheral, and wedge-shaped. Subcapsular hematomas are elliptical hemorrhages interposed between the renal capsule and the kidney parenchyma.
Major injuries comprise approximately 10% of renal injuries and include lacerations that extend from the cortex to the collecting system, multiple renal lacerations (known as shattered kidney), segmental infarcts, and vascular injury to the renal pedicle (often with active hemorrhage).
Sudden deceleration can disrupt the ureteropelvic or ureterovesical junctions. Surgical mishap, ureteroscopy, gunshot, and penetrating trauma are other causes. Disruption of the collecting system is confirmed by detecting contrast medial to the kidney or along the course of the ureter, and injuries are classified as complete (avulsion) or incomplete (laceration). Consequences of delayed diagnosis include urinoma, abscess, and stricture. AAST grading for renal injury is seen in Table 6.3 .
All grade I and II injuries and 90% of grade III and IV injuries are managed conservatively. Grade V injuries usually require nephrectomy ( Fig. 6.5 ).
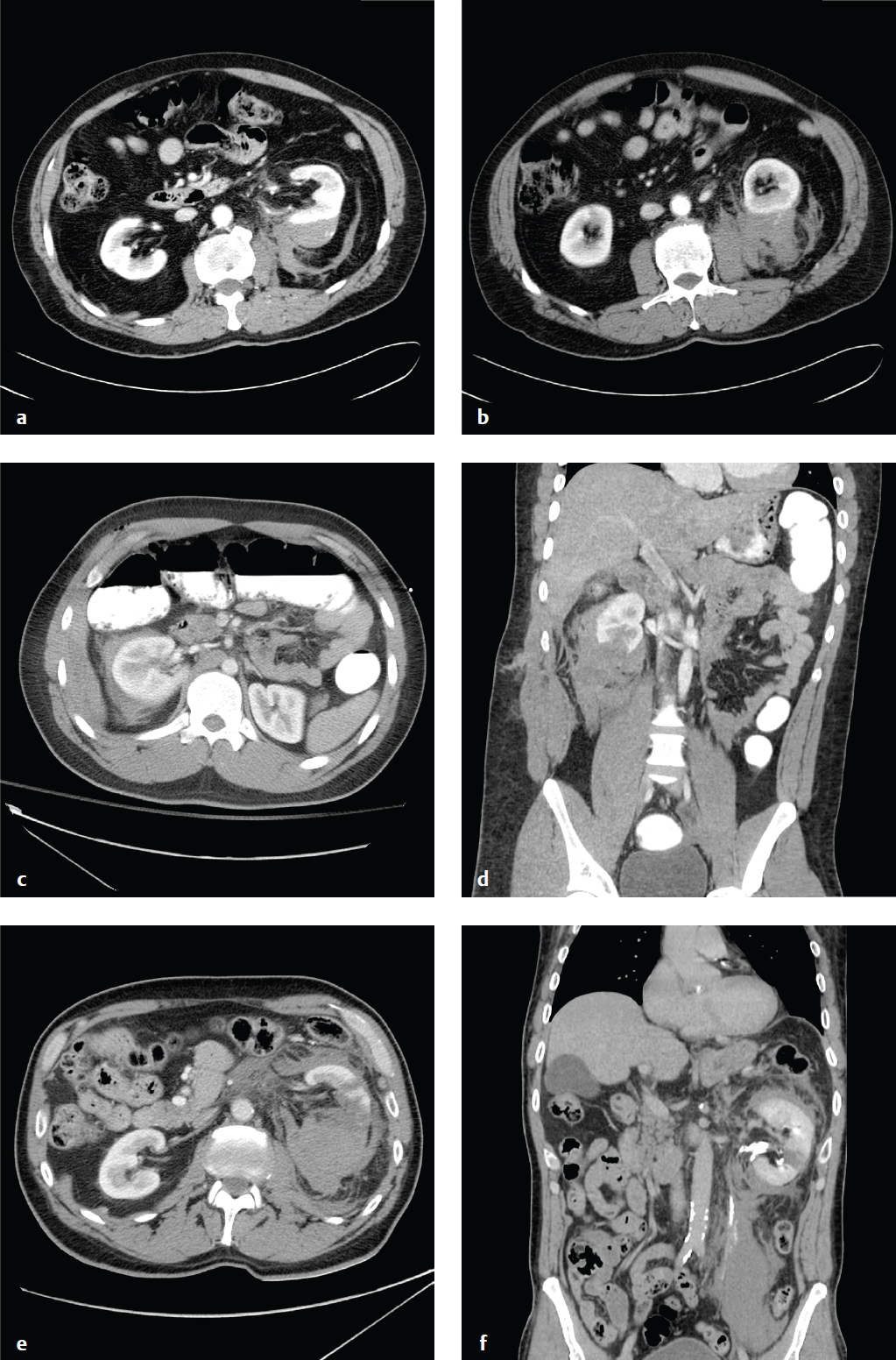
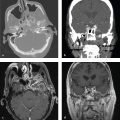
Pancreatic Trauma
Traumatic pancreatic injuries are rare, but missed or delayed diagnosis can result in significant morbidity and mortality. In blunt trauma with rapid deceleration, the midbody of the pancreas can be compressed against the spine and contused or transected. Unrestrained drivers and bicyclists who hit handlebars are both at particular risk for this injury. Patients may have few symptoms at presentation, and initial CT scans can even be normal. However, even in apparently minor injuries, symptoms can develop after several days as digestive pancreatic enzymes are released into retroperitoneal tissues.
Ultrasonography is of limited utility in the acute setting for evaluation of pancreatic injuries, but if performed, it may show hypoechoic defects within the parenchyma. CT is much more sensitive for detection of pancreatic injuries and reveals focal or diffuse edema, low-attenuation lesions, peripancreatic fluid, fat stranding, retroperitoneal hematoma, or combinations of these findings.
One should specifically examine the main pancreatic duct to assess for tran-section, as such injuries often require endoscopic retrograde cholangiopancreatography (ERCP) with stent placement. In other cases of pancreatic injury, surgical intervention may be required.
Complications of pancreatic trauma include pancreatitis, fistula, retroperitoneal abscess, pseudoaneurysm, or pseudocyst formation ( Fig. 6.6 ).
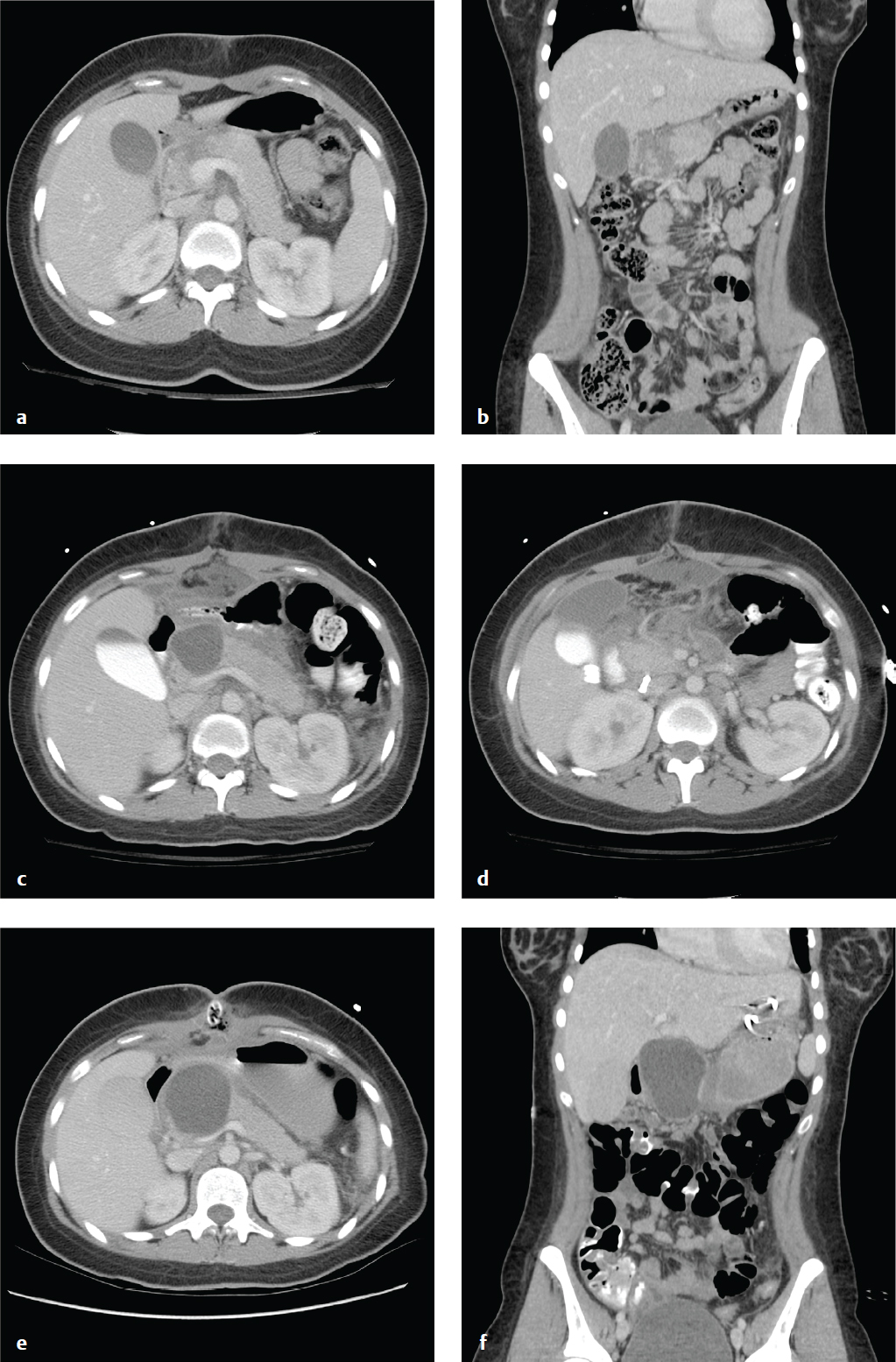
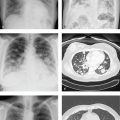
Shock Bowel and Small-Bowel Injury
The term shock bowel describes the intestinal CT findings seen in those trauma patients who are initially hypotensive and hypovolemic. Shock bowel is characterized by diffuse small-bowel wall thickening; fluid-filled, dilated small-bowel loops; and pronounced mucosal enhancement. The large bowel, in contrast, usually appears normal. Other visceral manifestations of hypotension include a flattened inferior vena cava (IVC), small-caliber aorta, and intense renal enhancement, the latter due to hypoperfusion. These generally resolve completely with effective fluid management.
Small-bowel injuries are often clinically subtle. Distracting injuries, intoxication, and depressed consciousness limit the sensitivity of physical examination. Free fluid may be missed on initial FAST examination, and peritoneal signs may be delayed. Witnessed or suspected abdominal impact and abdominal wall bruising should raise suspicion for bowel contusion or other gastrointestinal injury.
CT has decreased the historic need for exploratory laparotomies after blunt abdominal trauma and should be obtained in any stable patient. Focal small-bowel thickening indicates a probable contusion, and a discrete intramural or mesenteric hematoma may also be seen. Underdistended bowel can have a similar appearance.
The consequences of small-bowel laceration are blood loss and peritoneal contamination. Spillage of the intestinal contents into the abdomen ultimately produces a suppurative peritonitis that may take up to 8 hours to manifest clinically, or longer if the patient is unconscious or sedated. Concomitant mesenteric and visceral injuries are often present.
Pneumoperitoneum, oral contrast extravasation, bowel wall discontinuity, or mesenteric vascular extravasation indicate urgent operative exploration. In contrast to most solid organ injuries (e.g., spleen or liver), which are often observed, bowel perforation must be managed surgically.
Small amounts of free fluid, bowel wall thickening, mesenteric stranding, and bowel mucosal enhancement are suggestive findings, and surgery should be considered if two or more of these are present. In penetrating trauma, such as stab wounds, small amounts of extraluminal gas are nonspecific and can be due to air from the wound tract ( Fig. 6.7 ).
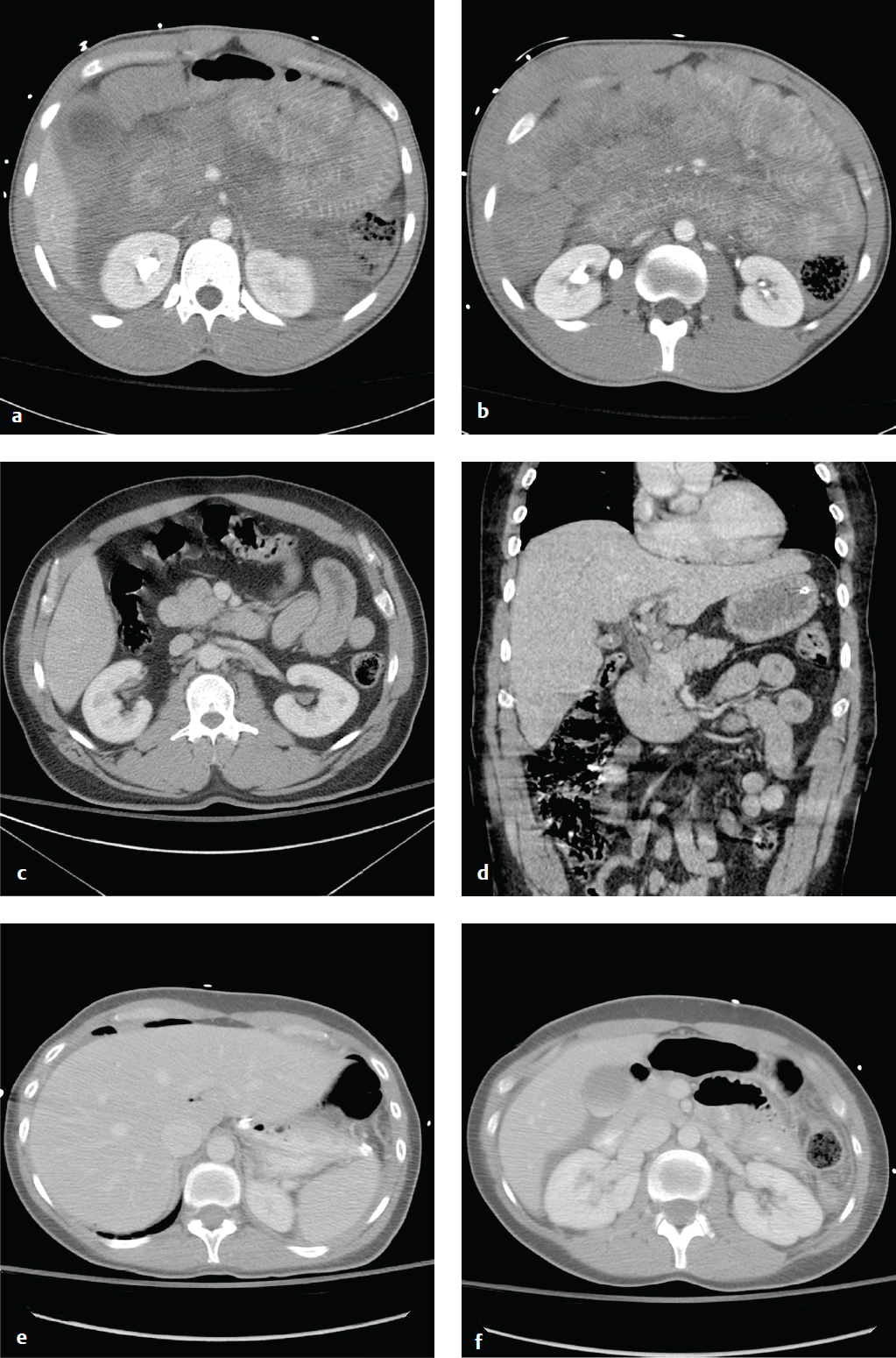
Bladder and Urethral Injury
Bladder injury in abdominal trauma is indicated by suprapubic pain, inability to void, gross hematuria, or microscopic hematuria greater than 25 red blood cells (RBC)/high-power field. Most injuries result from blunt external impact, frequently in conjunction with pelvic fractures.
Bladder rupture is classified as extra-peritoneal, intraperitoneal, or combined intra- and extraperitoneal. Most are extraperitoneal and result from shearing forces that deform the pelvic ring or direct puncture by pelvic bone fragments. If the bladder laceration is below the peritoneal reflection, urine extravasation is limited to the extraperitoneal space. Intraperitoneal extravasation results from disruption of the bladder dome, above the peritoneal reflection, and is often due to a direct blow to the lower abdomen in patients with a distended bladder. Intraperitoneal rupture tends to occur in intoxicated patients and belted passengers in motor vehicle accidents.
Bladder contusions and mural bladder hematomas are relatively benign and are diagnoses of exclusion. The bladder may appear normal or teardrop-shaped on cystography; management is conservative with observation for resolution of hematuria.
Standard abdominal CT scanning for trauma readily identifies perivesical fluid or pelvic fractures adjacent to the bladder, but it is insensitive for detection of small leaks. A dedicated CT or plain film cystogram, in which contrast is directly instilled into the bladder via a Foley catheter, more accurately excludes small bladder ruptures. In severe polytrauma requiring immediate laparotomy, cystography is usually bypassed, and the bladder is directly evaluated intraoperatively.
Urethral injuries, while uncommon, contribute to significant long-term morbidity from complications including impotence, stricture formation, urinary retention, or incontinence. They most commonly involve the posterior urethra and are seen less frequently in women, primarily because of shorter urethral length. In blunt trauma, posterior urethral injury is almost always associated with pelvic fractures. Anterior urethral injuries (penile, bulbar urethra) are more frequently due to blunt trauma to the perineum (straddle injuries) and may present years later with complications from stricture formation.
The male patient with pelvic trauma who presents with gross hematuria, blood at the urethral meatus, edema or hematoma of the perineum or penis, or with a “high-riding” or boggy prostate after a pelvic fracture is likely to have a urethral injury. In these patients, a retrograde urethrogram should be performed prior to the insertion of a Foley catheter, which could enlarge a laceration or contaminate a previously sterile hematoma ( Fig. 6.8 ).
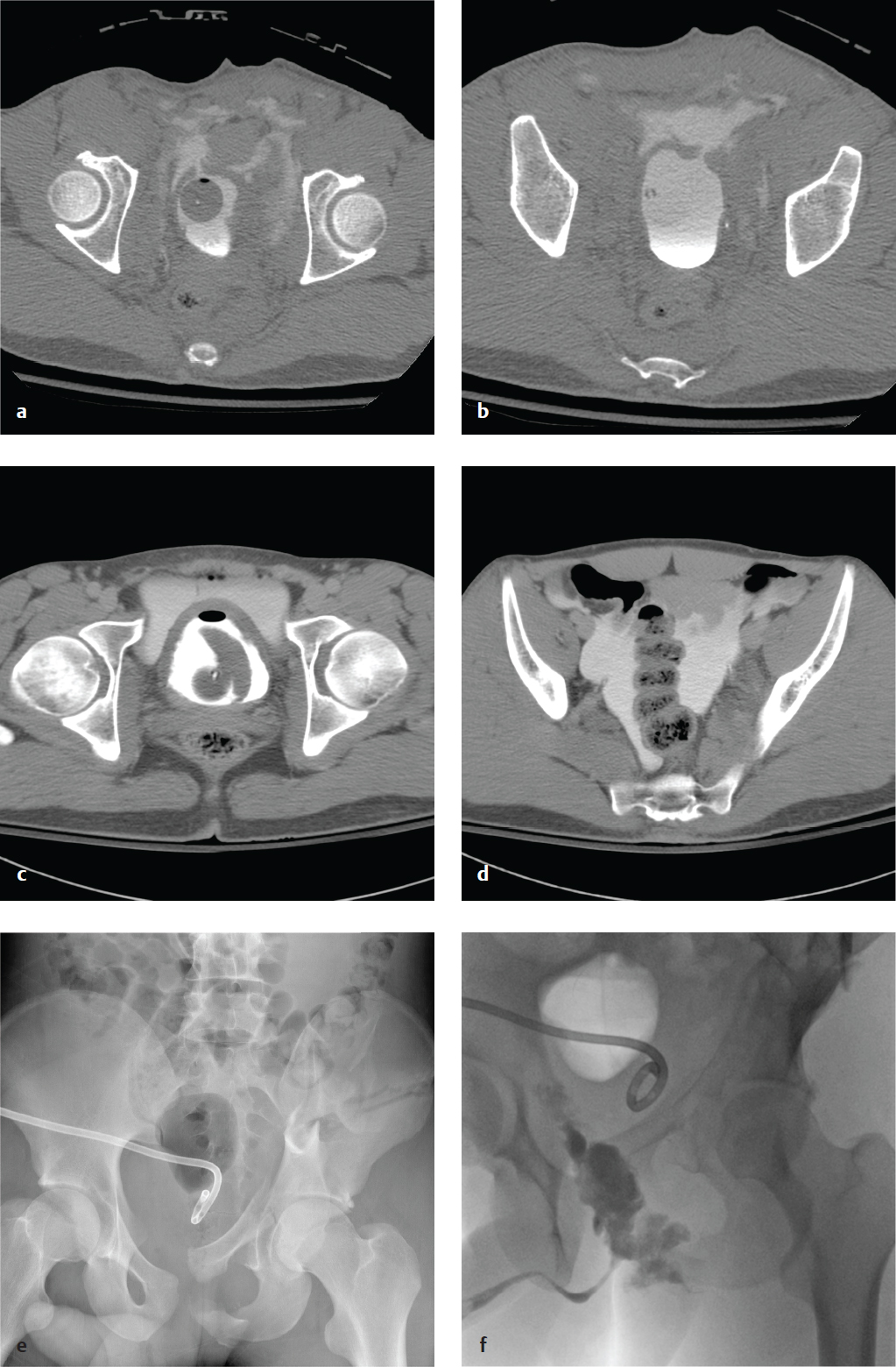
Gastric Ulcer/Gastric Outlet Obstruction
Causes of mechanical gastric outlet obstruction include pyloric or distal gastric malignancy, peptic ulcer disease, gastric polyps, diaphragmatic hernia, caustic ingestion and scarring, pancreatic pseudocysts, and bezoars. Delayed gastric emptying (gastroparesis) can result in distention and is most often due to diabetes, Parkinson disease, or multiple sclerosis. In both extrinsic and intrinsic obstruction, patients present with intermittent symptoms of vomiting and nausea, which worsen until obstruction is complete. Initially, patients tolerate liquids better than solid food. Progressive obstruction may lead to dehydration, electrolyte abnormalities, and malnutrition. Congenital pyloric stenosis occurs in infants aged 2–6 weeks who develop projectile vomiting after feeding.
Plain abdominal radiographs, upper GI contrast studies with Gastrografin or barium, and CT with oral contrast show gastric dilatation and contrast within an enlarged stomach and can identify gastric neoplasm, ulceration, and adenopathy.
Bezoars are concretions of ingested vegetable matter (phytobezoar) or hair (trichobezoar) that accumulate in the stomach and can form an obstructing cast. Poor gastric motility, as well as ingestion of indigestible plant fiber, can predispose to phytobezoars. Trichobezoars are seen in patients with psychiatric disorders due to compulsive hair pulling and ingestion. Radiographs show an enlarged gastric outline with a mottled intragastric mass. On barium studies, the bezoar is visible as an intraluminal filling defect that is not attached to the bowel wall. Delayed imaging is helpful, as barium is often trapped within the mass for hours. CT shows an intragastric mass with mixed density due to entrapped air and food in the bezoar′s interstices ( Fig. 6.9 ).
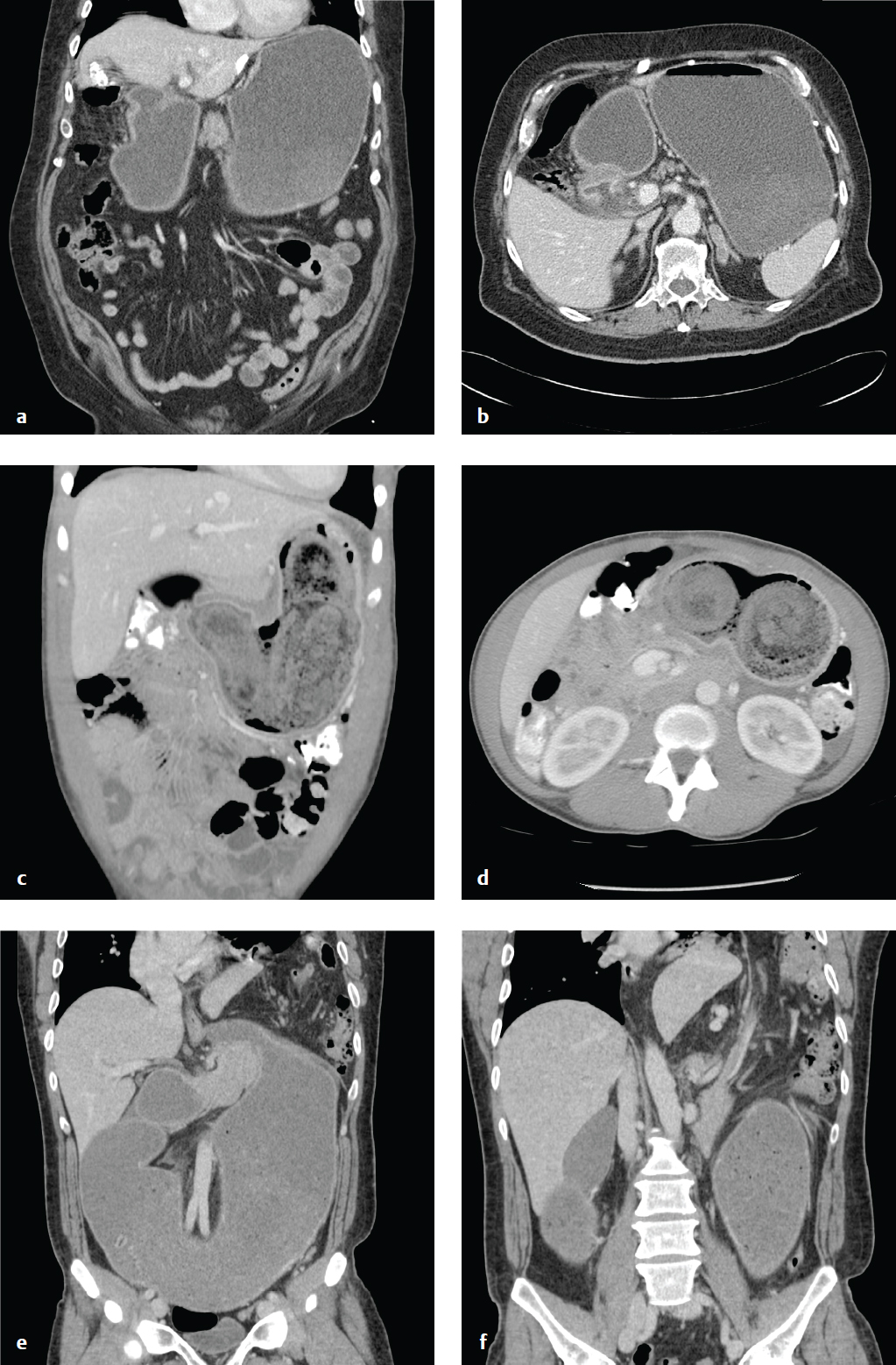
Duodenal Perforation
Duodenal perforation is usually a consequence of peptic ulcer disease, but it can also result from blunt abdominal trauma and can complicate endoscopy or other instrumentation. In peptic ulcer disease, duodenal perforation is two to three times as common as gastric perforation. Intestinal contents can spill into the peritoneal cavity (free perforation) or can be contained by adjacent organs. Diffuse peritonitis can complicate free perforation.
Free intraperitoneal air is detectable on plain radiographs in about two-thirds of patients with bowel perforation. When visible, it appears as small lucent crescents below the diaphragm on upright radiograph or below the antidependent abdominal wall on decubitus or CT examinations. On supine radiographs, larger quantities of air may outline the falciform ligament as well as the inner and outer surfaces of the bowel wall (Rigler sign). The most common causes of nontraumatic intraperitoneal air on plain radiographs are perforated duodenal ulcer, perforated diverticulitis, and less commonly, perforated colon carcinoma. Recent abdominal surgery is another etiology of free air, but that should be evident from the patient′s history.
Abdominal CT sensitively identifies free air, intra-abdominal fluid collections, and any contrast extravasation, and it can often delineate the location and morphology of the perforated viscus. Upper gastrointestinal series can identify ulcers, luminal narrowing, or irregularity and extravasation ( Fig. 6.10 ).
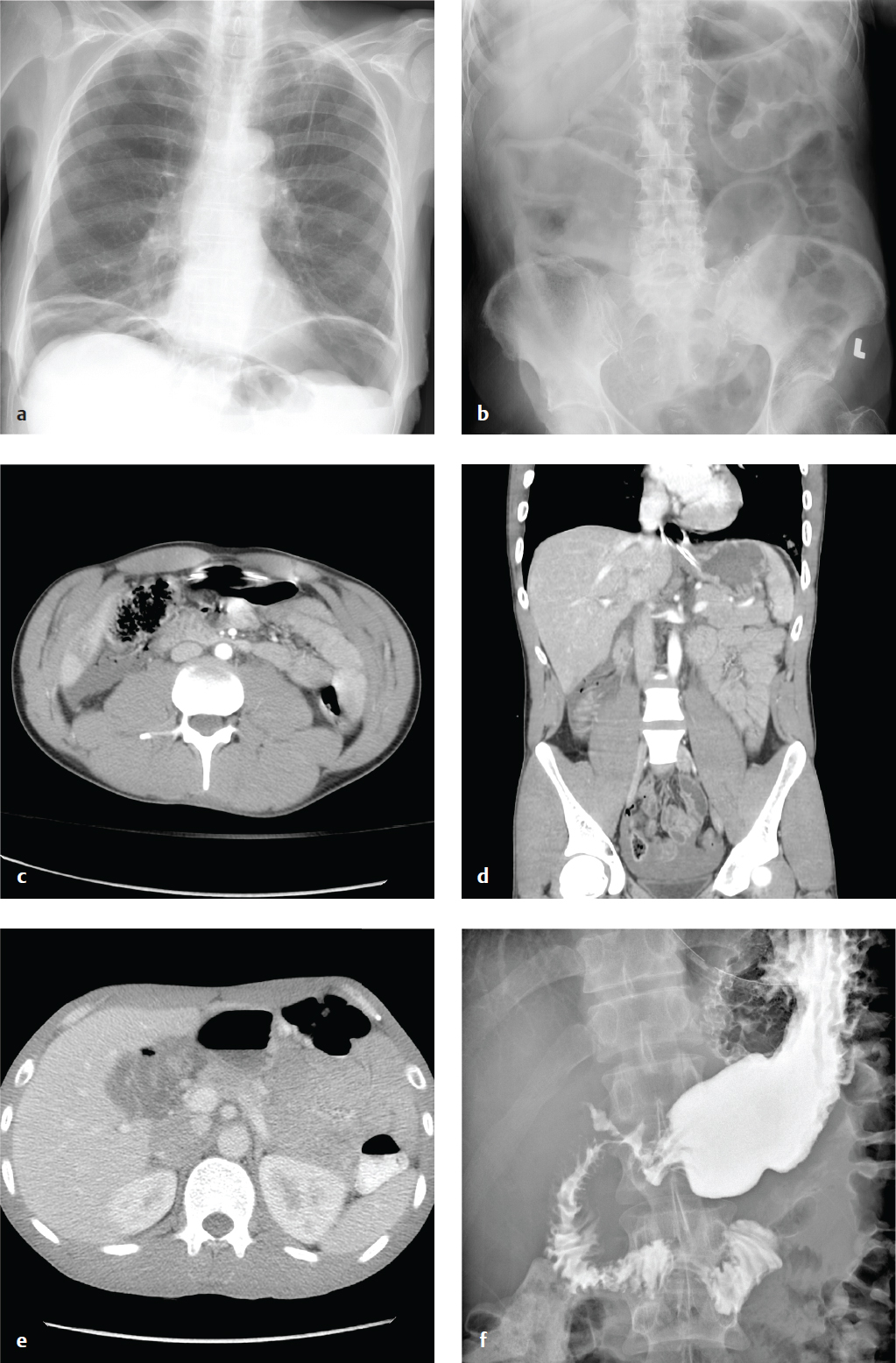
Acute Pancreatitis
Acute pancreatic injury, inflammation, or ductal obstruction can lead to intrapancreatic enzyme activation, disruption of pancreatic ducts, and leakage of pancreatic secretions with autodigestion of adjacent tissues. The most common causes of acute pancreatitis are chronic alcohol consumption and acute pancreatic duct obstruction from biliary stone disease. Less common causes include hyper-triglyceridemia, medications, and trauma.
The diagnosis is based on clinical and laboratory findings, severe upper abdominal pain in a patient at risk for pancreatitis, and elevated serum lipase. The role of CT in acute pancreatitis is to confirm the diagnosis, assess severity, and point to a cause (e.g., obstructing gallstone, pancreatic mass, cirrhosis). Imaging may be normal in mild or early disease, and its greatest value is in identifying subacute and late complications, such as necrosis, pseudocyst, and abscess formation.
Contrast-enhanced CT shows the edema and inflammation of acute pancreatitis as parenchymal enlargement, heterogenous attenuation, indistinct pancreatic margins, retroperitoneal fat stranding, and, if severe, peripancreatic fluid collections or frank pancreatic necrosis.
Ultrasound is less sensitive and specific for evaluating the pancreas than is CT, but it can be useful for detecting or following peripancreatic collections. When imaged by ultrasound, acute pancreatitis appears as an enlarged, hypoechoic pancreas, sometimes with an adjacent fluid collection.
CT grading (Balthazar) is seen in Table 6.4 . Patients with grades A through C have a 0% mortality and 4% complication rate, whereas patients with grade D or E pancreatitis have a 14% mortality and a 54% complication rate ( Fig. 6.11 ).
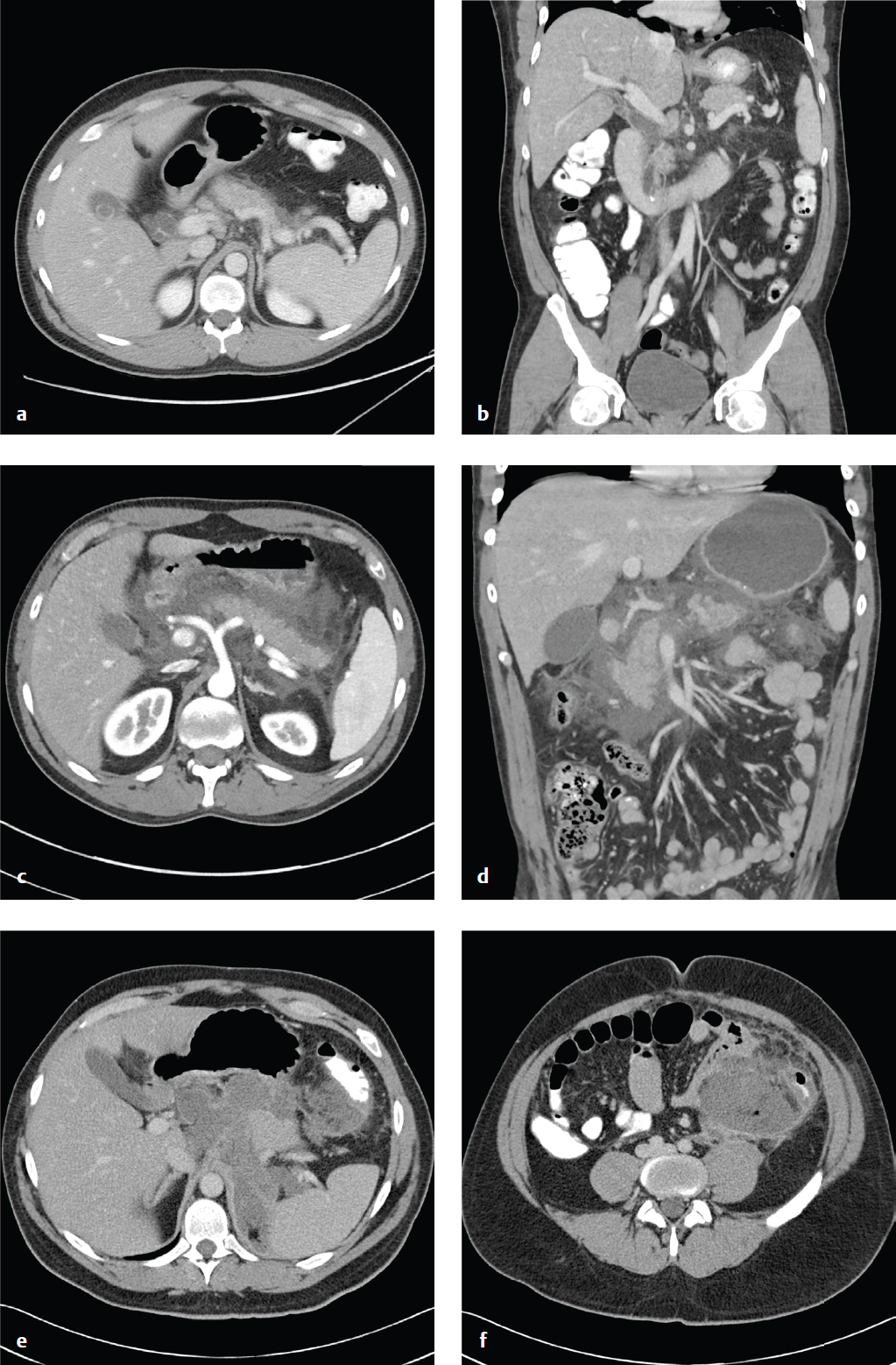
Pancreatic Necrosis and Chronic Pancreatitis
Pancreatic necrosis is one of the severe complications of acute pancreatitis and is associated with increased morbidity and mortality. Inflammation severe enough to cause cell death and liquefactive necrosis typically develops 24 to 48 hours after symptom onset. Consequently, CT scans obtained in the first 12 hours may be falsely reassuring. Contrast-enhanced CT 3 days after symptom onset will more accurately detect necrosis than studies obtained at initial presentation will.
In severe pancreatitis, a pseudocyst forms when necrotic tissue is sequestered within a fibrous capsule over a period of 3 to 4 weeks. The contained necrotic tissue is prone to infection and abscess development. There are three potential outcomes for pancreatic necrosis: resolution, formation of noninfected pseudocyst, or abscess.
Portions of the pancreas that fail to enhance after the administration of intravenous contrast are considered necrotic. Intra- or peripancreatic gas bubbles may also be seen. Pancreatic necrosis may be accompanied by peripancreatic fat necrosis, which appears as a heterogenous fluid collection adjacent to the pancreas.
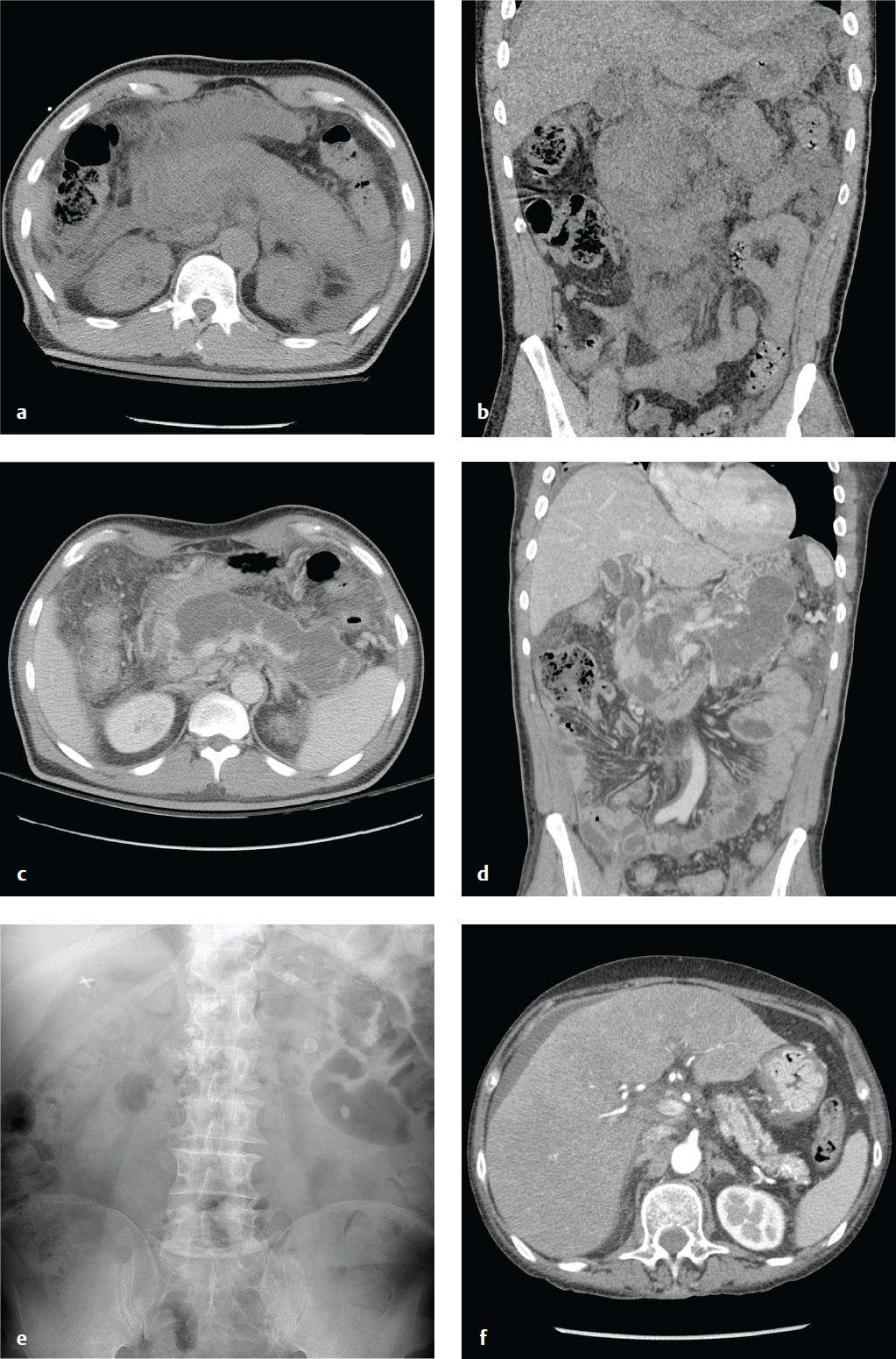
Chronic pancreatitis is the consequence of continuing, chronic pancreatic inflammation with eventual glandular fibrosis, atrophy, and dystrophic calcification. Patients report a long history of intermittent epigastric pain that radiates to the back or recurrent episodes of acute pancreatitis. With sufficient pancreatic damage, the gland′s endocrine and exocrine functions fail, leading to malabsorption and diabetes. In contrast to the laboratory abnormalities seen in acute pancreatitis, lipase and amylase may be normal or only mildly elevated.
Abdominal or chest radiographs often show punctate or coarse upper abdominal calcifications distributed transversely across the epigastrium. These are primarily intraductal, located either within the main pancreatic duct or in the small pancreatic duct radicles. CT and ultrasound may show focal enlargement or atrophy of the gland, parenchymal calcifications, and pancreatic duct dilatation with ductal width greater than 5 mm at the head and 2 mm in the body and tail ( Fig. 6.12 ).
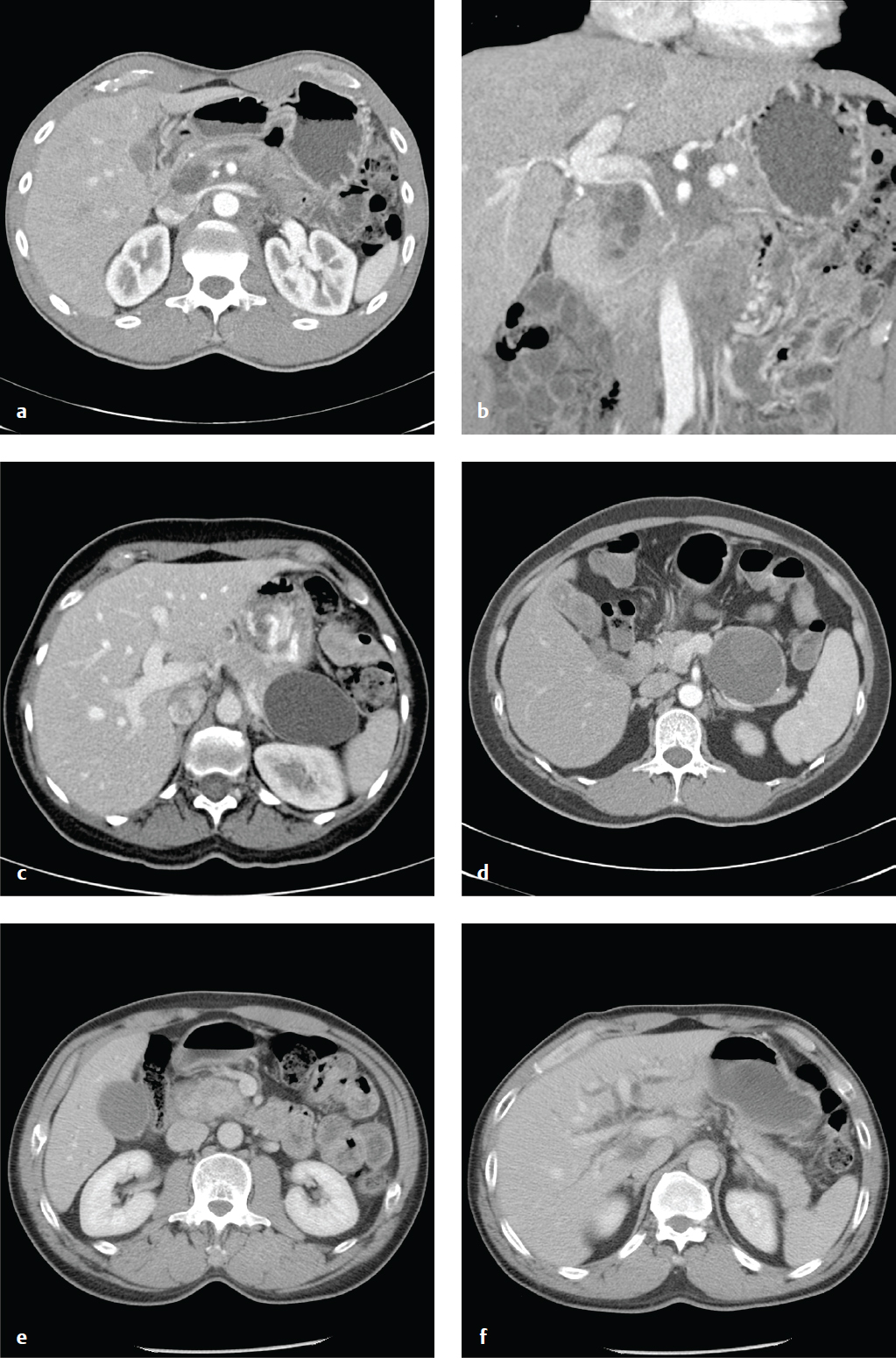
Pancreatic Masses and Pancreatic Adenocarcinoma
Pancreatic cysts are usually asymptomatic and incidentally discovered. Most of these are benign or indolent and include serous cystadenomas, mucinous cystic neoplasms, intraductal papillary mucinous neoplasms (IPMN), and pseudocysts. Ductal adenocarcinomas represent about 2.5% of incidentally discovered pancreatic cysts, and mucinous neoplasms, which have a malignant potential, represent about 55%.
Simple cysts < 2 cm should be reimaged in one year and, if stable, are considered benign.
Simple cysts 2–3 cm (and those that have grown from < 2 cm) should be characterized by MRI. Uncharacterized masses should be followed yearly. Cysts consistent with branch-duct IPMNs should be followed every 6 months for 2 years and annually thereafter. Cysts consistent with serous cystadenomas should be followed every 2 years and resection considered if they grow to > 4 cm.
Cysts > 3 cm should be characterized by MRI; most should be considered for cyst aspiration and resection depending on risk and comorbidities. Serous cystadenomas should be considered for resection when they reach 4 cm.
Solid pancreatic masses include true neoplasms, most notably adenocarcinoma and neuroendocrine tumors, and nonneo-plastic conditions that can mimic tumors, such as autoimmune pancreatitis, intrapancreatic spleen, or sarcoidosis. Solid tumors and cysts with solid components should generally be resected.
Pancreatic adenocarcinoma is the most common primary pancreatic malignancy and affects patients in the seventh and eighth decades who typically present with pain, jaundice, and weight loss. Unfortunately, most have unresectable disease at the time of diagnosis, due to hepatic and peritoneal metastases. Five-year survival is ~ 5%. Thin-section CT in both arterial and portal venous phases is the best technique for evaluating solid pancreatic tumors, and most will appear as low-attenuation lesions within the normally enhancing pancreas. Criteria for resectability include lack of distant metastases; clear fat planes around the hepatic, celiac, and superior mesenteric arteries; and tumor that does not abut the portal or superior mesenteric veins ( Fig. 6.13 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree