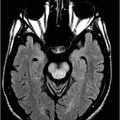
Axial non-enhanced CT scan of the head through the level of the lateral ventricle.
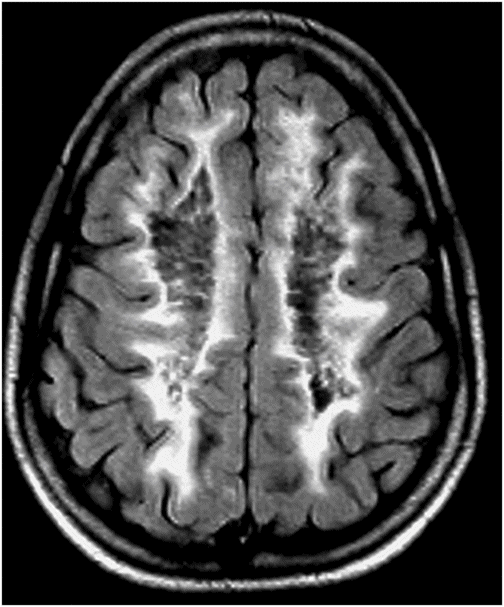
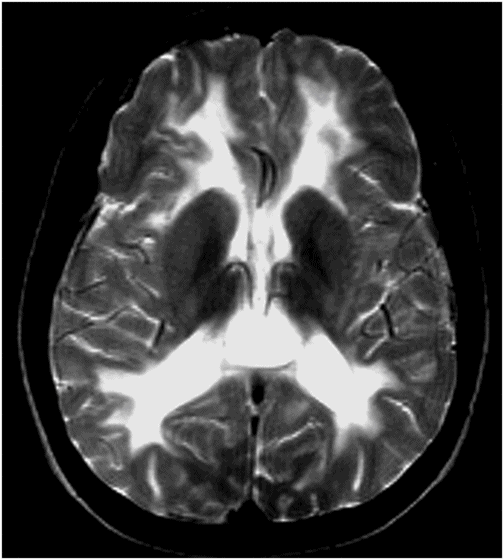
Axial FLAIR image of the brain through the centrum semiovale.
Vanishing White Matter Syndrome
Primary Diagnosis
Vanishing white matter syndrome
Differential Diagnoses
Metachromatic leukodystrophy (MLD)
Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL)
X-linked adrenoleukodystrophy (XLA)
Acyl CoA enzyme deficiency (ACED)
Megalencephalic leukoencephalopathy with subcortical cysts (MLSC)
Imaging Findings
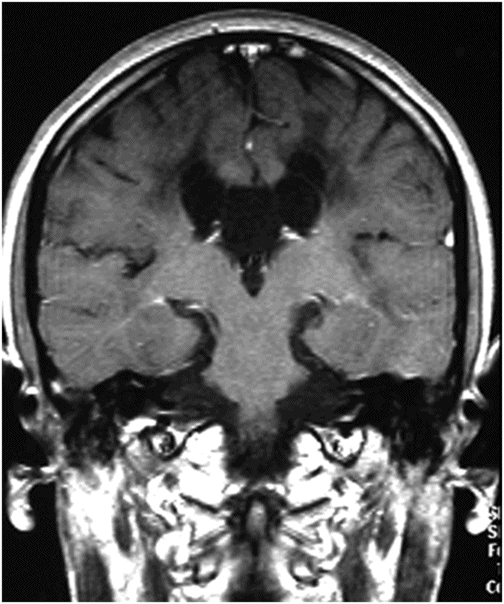
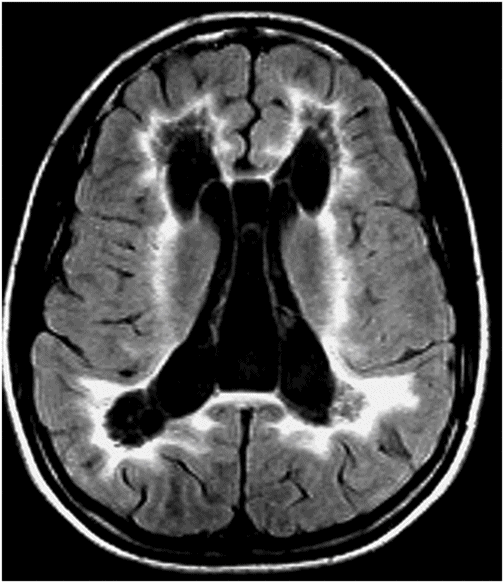
Fig. 60.1: Axial unenhanced CT scan demonstrated diffuse, bilateral, symmetric periventricular, deep and subcortical white matter marked hypodensity without mass effect. Fig. 60.2: Axial T2WI demonstrated diffuse, bilateral, symmetric periventricular, deep and subcortical white matter marked T2 hyperintensity without mass effect. Note that cortical gray and deep gray matters are spared. Fig. 60.3: Axial FLAIR image at the level of the centrum semiovale demonstrated thin, transversely oriented stripes within the rarefied white matter, diffuse white matter loss, and small capitations. Fig. 60.4: Enhanced coronal T1WI showed no enhancement in the abnormal white matter. Fig. 60.5: Axial FLAIR image of the brain (6-month follow-up) showed marked periventricular and deep white matter loss, with cavitation of the periventricular white matter that had progressed since the previous scan.
Discussion
A history of normal neurologic development in a young child, before the appearance of relatively acute-onset cerebellar-predominant neurologic signs and symptoms, following a febrile illness, which gradually progressed over time, is a typical clinical presentation of vanishing white matter (VWM) and rules out other congenial leukodystrophies such as MLD, LBSL, XLA, and ACED. Worsening T2 hyperintensity that predominantly involves the central white matter, with progressive rarefaction of the white matter structure, as evidenced by gradual lowering of the FLAIR signal over time, is a typical imaging finding of VWM. The thin transversely oriented stripes within the rarefied white matter further confirm the diagnosis of VWM.
The progressive rarefaction of white matter with central, transversely oriented stripes is not a characteristic feature of MLD, LBSL, XLA, and ACED. In addition, in MLD and LBSL, normal complete myelination is never noted, and patients present at a much earlier age. Moreover, the classic tigroid pattern of MLD is absent, further excluding MLD as a diagnostic option. The classic imaging feature of XLA, posterior, predominant white matter changes with diffusion restriction and enhancement at the advancing edge, are absent in this patient. Cyst formation is typical in MLSC but the cystic changes have been described in the anterior temporal lobe and frontoparietal subcortical white matter, rather than periventricular white matter. Additionally, in MLSC, peripheral white matter is more severely involved compared to the central white matter involvement, as noted in this patient.
Also known as childhood ataxia with CNS hypomyelination, VWM is a rare autosomal recessive CNS disorder. Despite affecting patients from all age groups, VWM is one of the most prevalent inherited childhood leukoencephalopathies and is caused by a mutation on chromosome 3q27.
The disease classically presents between two and five years of age. Patients have a chronic, progressive, and episodic course, beginning during infancy or early childhood, with a chronic progressive cerebellopyramidal syndrome and spasticity. Cerebellopyramidal symptoms can progress to extensive neurologic deterioration that is precipitated by minor stress conditions, such as infections or mild head trauma.
Along with the disease progression, individuals may experience hypotonia, irritability, vomiting, and epilepsy. Patients also may show consciousness impairment, which may vary from somnolence to coma, and occasionally death. The disease progression is heterogeneous with a wider clinical spectrum related to age at onset that is inversely related to clinical severity.
Magnetic resonance imaging in VWM typically shows diffuse and symmetric signal abnormalities of the cerebral white matter, which becomes increasingly rarefied and subsequently cystic. Typically, patients present confluent and symmetric abnormalities in the periventricular and deep white matter. In young patients, myelination may be delayed. Reactive astrocytosis along the medullary veins leads to radial stripes that stretch from the ventricular wall to the subcortical regions, which is another typical imaging feature. Subcortical U fibers, the outer part of the corpus callosum, the internal capsules, and anterior commissure are typically spared.
Contrast enhancement has not been reported in VWM patients. Longitudinal follow-up imaging studies usually demonstrate progressive replacement of white matter by cystic areas. In cerebral white matter, DWI increased diffusivity is apparent while proton spectroscopy demonstrates progressive reduction and eventual disappearance of all major metabolites, with the accumulation of glucose and lactate at CSF-like foci. The cerebellar white matter may also be affected in VWM (typically without cystic degeneration). In the brainstem, abnormal signal intensity may be detected, particularly in the pontine central tegmental tracts.
Typically, abnormalities of the inner rim of the corpus callosum are noted. In the early stages of VWM, MRI does not necessarily display diffuse cerebral white matter involvement, rarefaction, or cystic degeneration. If MRI abnormalities do not meet the criteria for VWM, the diagnosis should be considered if the inner rim (the callosal-septal boundary) is affected.
Vanishing white matter provokes widespread white matter abnormalities, not only in the anomalous-appearing white matter detected on MRI, but also in the normally appearing brain parenchyma, including the gray matter. Typical pathologic findings of VWM include increasing white matter rarefaction and cystic degeneration, appearance of oligodendrocytic densities with highly characteristic foamy oligodendrocytes, presence of meager astrogliosis with dysmorphic astrocytes, and loss of oligodendrocytes by apoptosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree