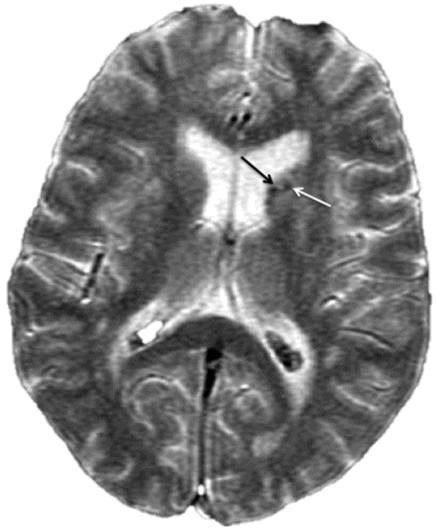
Axial T1WI through the basal ganglia.
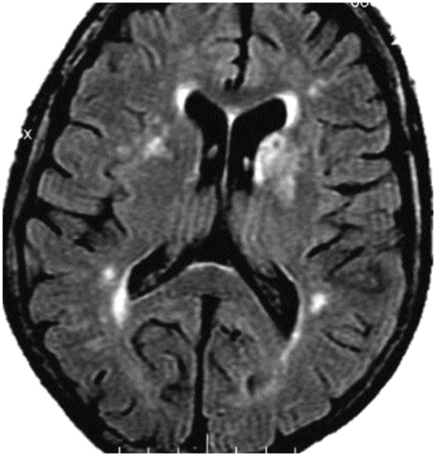
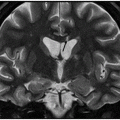
Axial FLAIR through the basal ganglia.
Hyperglycemic Hemiballismus and Hemichorea
Primary Diagnosis
Hyperglycemic hemiballismus and hemichorea
Differential Diagnoses
Chronic hepatic encephalopathy
Long-term total parenteral nutrition/hyperalimentation
Methanol toxicity
Metabolic abnormalities
Imaging Findings
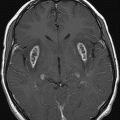
Fig. 64.1: Axial T1WI sequence through the level of the basal ganglia demonstrates T1 hyperintensity, predominantly involving the striatum (caudate nucleus and the putamen) on the left side. Subtle T1 hyperintensity is noted in the left globus pallidus. The left thalamus is normal. No abnormality is noted in the right basal ganglia or in the right thalamus. Fig. 64.2: Axial FLAIR image through the same level demonstrates abnormal T2 signal involving the left striatum, in the areas of T1 hyperintensity. Fig. 64.3: Axial GRE image through the same level demonstrates subtle punctate areas of hypointensity at the head of the left caudate nucleus (arrows). No hypointense signal is noted in the left putamen or globus pallidus.
Discussion
Presentation of a patient with a history of uncontrolled diabetes mellitus, in conjunction with typical, relatively acute-onset hemichorea, hemiballismus movements, and the presence of unilateral T1 hyperintensity involving the striatum on imaging is diagnostic of hyperglycemic hemiballismus and hemichorea (HHH).
The demonstrated imaging unilaterality and laboratory findings in this case exclude other processes such as hepatic encephalopathy (HE), hyperalimentation, or methanol toxicity. In addition, in hyperalimentation and chronic HE, T1 shortening and hyperintensity is typically seen in the globus pallidus, not the striatum, as seen in this patient. Methanol toxicity predominantly or exclusively involves bilateral putamina, but with infarction and diffusion restriction, which is absent in this case. T1 hyperintensity in the basal ganglia can also be seen in chronic HE, and in manganese toxicity secondary to long-term parenteral nutrition. Usually in these conditions, the abnormality is bilateral, often symmetric, and more commonly involves the putamen, in contrast to the caudate nuclei.
Hemiballism-hemichorea (HH) is a clinical spectrum of continuous, non-patterned, and involuntary movements involving one side of the body. A focal structural or vascular lesion in the contralateral basal ganglia, particularly striatum, is the most common cause. Metabolic derangements (such as non-ketotic hyperglycemia or hyperthyroidism) and infectious diseases (e.g., HIV infection) can also present with HH, in the absence of an identifiable lesion at the basal ganglia on imaging. Hyperglycemic hemiballismus and hemichorea is a recently described clinicoradiologic syndrome characterized by striatal (caudate and putaminal) T1 hyperintensity (contralateral to the abnormal movements) and is typically seen in older females of Asian descent with a history of uncontrolled diabetes mellitus. Although HHH has been described in patients with high blood glucose at presentation, presentation with normal glucose level has also been described. Hyperglycemic hemiballismus and hemichorea may be the presenting symptom of type 2 diabetes mellitus. The symptoms often reverse on achieving a euglycemic state; however, they may persist for years.
Typical imaging findings of HHH include unilateral T1WI hyperintensity in the striatum, hypointensity on T2WI, and an unremarkable GRE sequence. Hyperdensity in the involved areas on CT and T2 isointensity have also been described. The pathophysiologic basis of the T1 hyperintensity is unclear. Many hypotheses have been proposed including petechial hemorrhage, myelinolysis, and calcium and manganese deposition. Bilateral involvement has also been reported in the literature. The striatal hyperintensity has been reported to be reversible in some patients. There are two atypical features in this patient on imaging: T2 hyperintensity and hemorrhage on GRE. Magnetic resonance spectropscopy demonstrates low NAA/Cr ratio, high choline/Cr ratio, and associated lactate peak, indicating energy depletion and neuronal dysfunction. Single photon emission computed tomography and PET studies have revealed reduction of striatal blood flow and metabolism.
The HH pathogenesis triggered by hyperglycemia is unclear. Possible etiologic explanations including: 1) hyperviscosity due to hyperglycemia (causing disruption of the blood-brain barrier and leading to intracellular acidosis); 2) lack of acetoacetate in a non-ketotic state to form GABA in thalami or striatum; 3) increased sensitivity of dopamine receptors in postmenopausal women; 4) petechial hemorrhages; and rarely, 5) striatal infarction have been described by some in the literature. Typical histopathologic findings are gliosis, selective neuronal loss, and gemistocyte accumulation without any evidence of hemorrhage or infarction.
Symptoms mostly resolve in response to treatment of the hyperglycemia. In up to 20% of cases, the symptoms persist even after three months. Although severity of the symptoms decrease, compared to severity at presentation, in some patients the symptoms may persist. In patients refractory to treatment of hyperglycemia, treatment with dopamine-blocking agents, such as topiramate, may prove efficacious.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree