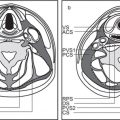
8 Suprahyoid Neck(Table 8.4 – Table 8.7)
Disease | CT Findings | Comments |
Congenital/developmental lesions | ||
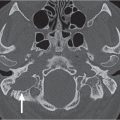
First branchial cleft cyst | Well-defined, unilocular, ovoid to round, nonenhancing or rim-enhancing fluid attenuation mass located periauricular (anterior, below, or posterior to pinna [type I]) or immediate periparotid (superficial, parotid, and parapharyngeal spaces [type II]); may have a direct connection with the external auditory canal. If infected, the wall may thicken and enhance, and the cyst content develops higher attenuation. | First branchial cleft cysts are very rare (only 8% of all branchial cleft remnants). Both children and adults may be affected. Most commonly seen in middle-aged women with recurrent abscesses around the ear or at the angle of the mandible; unresponsive to treatment. May be associated with branchial cleft fistula or sinus. Otorrhea commonly occurs if the cyst drains into the external auditory canal. |
Infantile hemangioma (capillary hemangioma) | Solitary, multifocal or transspatial, lobular soft tissue mass, homogeneous and isodense with muscle. No calcifications. Infantile hemangiomas can be well circumscribed, replacing the parotid gland, or can be infiltrative and might involve periparotid structures. Contrast enhancement is uniform and intense. Size, enhancement, and vascularity of tumor regress in involutional phase with internal, low-density fat. | By far the most common parotid gland lesion in the first year of life. A cutaneous hemangioma may also be found overlying the parotid region as an associated finding. True capillary hemangiomas are neoplastic conditions; typically display a rapid proliferation phase during the first year of life followed by an involution phase with fatty replacement. Occur predominantly in girls. Infantile hemangiomas are high-flow lesions during their proliferative phase, low-flow lesions in their involutional phase. Hemangiomas may enlarge rapidly due to bleeding within. |
Lymphatic malformation (lymphangioma, cystic hygroma) | Lymphatic malformations can involve the parotid gland directly or by local extension. Tend to appear as cystic masses filled with homogeneous low-attenuation material and without contrast enhancement. Cysts usually have thin walls, and most commonly there are multiple intercommunicating cystic components. Infected lesions show higher attenuation, an enhanced thickening of the cyst wall, and infiltration of the adjacent soft tissues. In the case of hemorrhage, fluid–fluid levels may be observed. | Lymphatic malformations represent a spectrum of congenital low-flow vascular malformations, differentiated by size of dilated lymphatic channels. May be sporadic or part of congenital syndromes (Turner, Noonan, and fetal alcohol syndrome). Macrocystic lymphatic malformation is the most common subtype. Sixty-five percent are present at birth; 90% are clinically apparent by 3 y of age, with the remaining 10% present in young adults. In children, the most common location is the posterior cervical space, followed by the oral cavity. In adults, lymphatic malformations are rare, may be posttraumatic, and are more commonly seen in the sublingual, submandibular, and parotid spaces. Often found in multiple contiguous spaces (transspatial). Other cystic lesions of the parotid gland are sialoceles and dermoid, epidermoid, mucus retention and sebaceous cysts. |
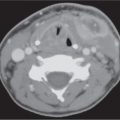
Polycystic disease of the parotid glands | Bilateral, markedly enlarged parotid glands with small areas of decreased density producing a mildly inhomogeneous appearance. | Rare inherited familial disorder, affecting predominantly women (gender linked?) with bilateral, enlarged, nontender parotid swelling, due to multiple cystic areas replacing the parenchyma, present for months or years. Hormonal changes (e.g., during pregnancy) may cause expression or exacerbation. |
Inflammatory/infectious conditions | ||
|
| Acute viral, bacterial, and calculus-induced parotitis are the most common salivary gland abnormalities. |
Acute viral parotitis | Usually bilateral. The involved parotid glands are somewhat enlarged, dense, and enhance slightly. Submandibular and sublingual glands may also be involved. | Mumps is the most frequent acute viral infection of the parotid glands. Other viral etiologies are Coxsackie viruses, parainfluenza viruses, influenza virus type A, herpes virus, echovirus, and choriomeningitis virus. Viral infection is associated with systemic infection. |
Acute suppurative parotitis and abscess | Usually unilateral. Diffuse swollen, enhancing parotid gland with focal area of rim-enhancing fluid attenuation. The parotid duct may be dilated with or without calculus. There are secondary inflammatory changes in the overlying subcutaneous fat and skin. Intraparotid and periparotid lymph nodes may be involved in the inflammatory reaction. The abscess may extend into the masticator and parapharyngeal spaces or upper neck. | Bacterial infection with unilateral cheek swelling associated with fever, chills, and elevated WBC. Most common bacterial organisms are Staphylococcus aureus (50%–90%), Streptococcus viridans, Haemophilus influenzae, Escherichia coli, anerobes, and Streptococcus pneumoniae. |
Benign lymphoepithelial lesions (BLLs) of human immunodeficiency virus | Usually bilateral parotid gland enlargement with multiple, predominantly superficial intraglandular masses with a homogeneous cystic appearance, hypodense (10–25 HU) with thin rims of enhancement, varying in size from a few millimeters to several centimeters (rare). Intraparotid lymph nodes may also be enlarged. Often with coexistent diffuse homogeneous cervical lymphadenopathy and adenoidal, faucial, and lingual tonsillar hypertrophy. No calculi are identified in the glands or ducts. | Lymphoepithelial cysts in the parotid gland may be secondary to incomplete ductal obstruction by periductal lymphocytic infiltration or arise within the intraparotid lymph nodes. Occurs with painless facial swelling in HIV-positive patients (CD4 level usually below 500/mL) and may be the first manifestation of the infection. |
Chronic recurrent parotitis Unilateral, diffusely enlarged parotid gland, often slightly denser than normal, with or without dystrophic calcifications. Cystic changes in the gland and dilated Stensen duct with or without calculi may be evident. Irregular intraglandular sialectasis can only be appreciated on CT sialogram. | Chronic inflammatory disease of the salivary glands can result in loss of parenchymal as well as fatty matrix and consequent shrinkage of the gland. | Strictures, calculi, or both within the main salivary gland duct may result in chronic sialodochitis and sialadenitis. Chronic recurrent sialadenitis is clinically characterized by recurrent diffuse or localized painful swelling of the salivary gland. |
Granulomatous parotitis | Unilateral or, less commonly, bilateral diffuse enlargement of the parotid gland, with multiple small nodular “foamy” densities distributed throughout the gland or a solitary mass. There is often an associated cervical lymphadenopathy. | The granulomatous diseases that may involve the major salivary glands include sarcoidosis, tuberculosis, atypical mycobacterial infection, syphilis, cat scratch fever, toxoplasmosis, and actinomycosis. These diseases may affect the intraparotid or juxtaglandular lymph nodes or the gland parenchyma directly. Presents usually as a nontender, painless, chronic enlargement of the gland. |
Sjögren syndrome | In the early stage of disease, CT demonstrates nonspecific enlargement of the parotid glands. In later stages, CT may demonstrate an increased density of the glands, a tiny honeycomb glandular appearance, or an inhomogeneous pattern with cysts, solid nodules, and premature fat depositions with heterogeneous enhancement and punctate calcifications. There is no diffuse cervical lympadenopathy. Parotid sialogram reveals a normal central duct system and numerous globular collections of contrast material, 1 to 3 mm in diameter, uniformly scattered throughout the gland. | Sjögren syndrome is an autoimmune disease with chronic inflammation of the exocrine glands that occurs either alone (primary Sjögren syndrome) or with any of several connective tissue diseases (secondary Sjögren syndrome). The adult form predominantly affects women over 40 y of age with keratoconjunctivitis sicca, xerostomia, and xerorhinia. Associated with clearly increased risk of developing non–Hodgkin lymphoma in intra- or extraparotid sites. |
Benign neoplasms | ||
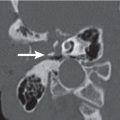
Benign mixed tumor (pleomorphic adenoma) | Small tumors appear as sharply marginated, intraparotid, round or ovoid, isodense or hyperdense with parotid gland tissue, homogeneously mild to moderate enhancing mass. Large tumors (> 2 cm) present as lobulated, well-circumscribed, inhomogeneously enhancing mass (in two-phase CT, these tumors present with an increase in attenuation during the second phase), with areas of lower attenuation representing foci of necrosis, old hemorrhage, cysts, or fat. Benign mixed tumors are the most common salivary tumors to have calcifications or ossifications in the tumor matrix. A large, deep, pear-shaped mass may widen the stylomandibular notch and displace the parapharyngeal space anteromedially. | Benign mixed tumor follows the rule of 80s: 80% of parotid tumors are benign; 80% of benign parotid tumors are benign mixed tumors; 80% of parotid benign mixed tumors are in the superficial lobe; 80% of salivary gland benign mixed tumors are parotid; 80% of untreated benign mixed tumors remain benign. Most common parotid tumor; slow-growing, painless benign neoplasm in the cheek. Most common in Caucasians, rare in African Americans (M:F = 1:2; age range 30–60 y). |
Warthin tumor (papillary cystadenoma lymphomatosum) | Usually located in the posterior aspect of the tail of the parotid gland; appear as round to ovoid, smoothly marginated, strong and homogeneously enhancing soft tissue masses, measuring 2 to 4 cm, that contain no calcification. Cyst formations with mural nodule are common. In two-phase CT, these tumors present with a decrease in attenuation during the second phase. There is no evidence of cervical lymphadenopathy. | Nearly exclusive to the parotid and the most common parotid neoplasm to become manifest, either multiple in one gland, bilaterally, or as cystic lesions. Most commonly occurs in men. Smoking predisposes to parotid adenolymphoma. There is a peak incidence in the fifth to seventh decades. Clinical symptoms can be attributed to enlargement of the lesion. Malignancy developing is extremely rare. |
Oncocytoma | Similar in appearance to benign mixed tumor and solid Warthin tumor. | Rare tumor that occurs in the major salivary glands, exclusively in adults older than 50 y. |
Lipoma | Well-circumscribed, homogeneous mass, isodense to fat (–65 to –120 HU), without contrast enhancement. Angiolipoma is similar to ordinary lipomas except for associated angiomatous proliferation. CT demonstrates marked enhancement around the fatty components. | Lipomas represent ~1% of all parotid tumors, invade deeply into the intraglandular septa, and occur in all age groups. |
Facial schwannoma | Tend to occur along the facial nerve as well-defined, fusiform soft tissue mass with varying degrees of contrast enhancement. Some of the parotid facial nerve tumors may be extensive, multilobulated, with areas of cystic formation and inhomogeneous enhancement. | Facial schwannomas are usually solitary and manifest as a slowly enlarging, painless mass. These tumors can occur at any age, most commonly between 20 and 50 y, with a female predominance. |
Neurofibroma | Intraparotid, most often ovoid, well-demarcated, heterogeneous, low-density mass, solitary or multiple. Absence of contrast enhancement is conspicuous. Plexiform neurofibroma shows a usually large, illdefined, more infiltrative, heterogeneous mass with a mixed density pattern. A typical finding is the target sign with a punctate high attenuation centrally surrounded by peripheral low attenuation within multiple small tumor nodules. | Neurofibroma may arise from the facial nerve trunk or its branches and may therefore lie within the parotid gland. May be localized, solitary or multiple, or plexi-form. Multiple or plexiform neurofibromas are seen in patients with von Recklinghausen disease. |
Malignant neoplasms | ||
Mucoepidermoid carcinoma | Low-grade carcinomas may be benign in appearance with well-circumscribed, smooth borders. The ovoid mass is isodense to muscle. Cystic areas are occasional and focal calcifications rarely present. High-grade carcinomas demonstrate infiltrating margins, particularly when associated with adjacent soft tissue or muscle invasion and an inhomogeneous aspect. Malignant adenopathy is often present (levels 2 and 5; intra- and periparotid nodes). | Parotid tumors are uncommon neoplasms; 15% to 20% are malignant. Mucoepidermoid carcinoma is the most common malignant tumor of the parotid gland. It represents 10% of all salivary gland tumors and 30% of all salivary gland malignancies. There is a male predominance between the ages of 35 and 65 y (may also be seen in the pediatric population). Presents with a rock-hard parotid mass and associated pain or itching in the facial nerve distribution. Facial nerve paralysis is an ominous sign. Major salivary gland carcinoma: TNM classification Primary tumor: T1: Tumor < 2 cm without extraparenchymal extension T2: Tumor 2 to 4 cm without extraparenchymal extension T3: Tumor > 4 cm with or without extraparenchymal extension T4a: Tumor invades skin, mandible, ear canal, and/or facial nerve T4b: Tumor invades skull base and/or pterygoid plates and/or encases carotid artery Regional lymph nodes: N1: Single ipsilateral node < 3 cm N2a: Single ipsilateral node 3 to 6 cm N2b: Multiple ipsilateral nodes ≤ 6 cm N2c: Bilateral or contralateral nodes ≤ 6 cm N3: Node (s) > 6 cm Distant metastasis: M1: Distant metastasis |
Adenoid cystic carcinoma | Parotid mass, isodense to muscle with homogeneous enhancement. May be well defined or poorly defined, depending on grade. Prone to perineural spread on CN V and VII. | Second most common parotid malignancy (and the most common in submandibular, sublingual, and minor salivary glands), with slight female preponderance between the ages of 50 and 70 y. Squamous cell carcinoma, adenocarcinoma, acinic cell carcinoma (most common multifocal parotid malignancy), undifferentiated carcinoma, epithelialmyoepithelial carcinoma, and atypical carcinoid are much less common and cannot be distinguished on CT because margins, architecture, and CT density overlap. |
Non–Hodgkin lymphoma | CT may demonstrate unilateral or bilateral, solitary or multiple well-circumscribed, homogeneous, nonnecrotic, enlarged intraparotid lymph nodes with mild to moderate homogeneous enhancement, or a diffuse parotid infiltration with moderate to high density, with or without extension into adjacent spaces. Periparotid and cervical lymphadenopathy is often present. Leukemic infiltration of the parotid gland is indistinguishable from infiltrative lymphoma. | Primary malignant lymphoma arising from the parotid gland is rare, whereas secondary involvement is common. An increased incidence is noted in Sjögren syndrome. |
Intraglandular metastases | Single or multiple, unilateral or bilateral intra- and periparotid soft tissue masses, round or with infiltrating or invasive margins, often inhomogeneous with central necrosis. | Metastatic disease from a primary malignancy outside the parotid gland is a rare condition (4% of all salivary neoplasms, M:F = 2:1, seventh decade). Malignant melanoma and cutaneous squamous cell carcinoma (face, auricle, and scalp) account for the majority of lymphatic metastases to the parotid gland. Primary disease of the breast, lung, kidney, prostate, and gastrointestinal (GI) tract account for the majority of systemic metastases to the parotid gland. |
Miscellaneous lesions | ||
Sialosis or sialadenosis | Parotid disease is usually bilateral and symmetric, but it can be unilateral or asymmetric. The parotid glands are enlarged but may appear either dense or fatty, depending on the dominant pathologic change. | Metabolic or endocrine related salivary gland disorders with nonneoplastic, noninflammatory, nontender, chronic or recurrent enlargement of the major or minor salivary glands. May be associated with diabetes, cirrhosis, alcoholism, malnutrition, hormonal imbalance, and drugs. |








Disease | CT Findings | Comments |
Pseudomass | ||
Ectatic carotid artery | One or both internal carotid arteries may have a retropharyngeal course and are imposing as round enhancing structure in the widened retropharyngeal space. Bilateral tortuous internal carotid arteries migrating medially to touch in the midline of the retropharyngeal space are called “kissing carotids.” If the carotid artery folds sharply on itself, the appearance of an enhancing carotid space mass may be suggested. Sectional imaging, reconstructions, and CT angiography (CTA) easily define the vascular nature of the tubular, tortuous, elongated, sometimes dilated carotid artery. | A tortuous internal carotid artery can manifest as a pulsatile mass in the carotid triangle at physical examination. It can also present as a submucosal mass displacing the pharyngeal posterior wall. Increasing incidence of medial loop is seen with increasing age. |
Asymmetric internal jugular vein | The size of the internal jugular veins can be variable and asymmetric due to their reciprocal size relationship to the external jugular veins and positional and anatomical factors. The right internal jugular vein is usually larger than the left. | A wide range of normal variation exists in symmetry of the internal jugular veins, from complete absence of one vein to perfect bilateral symmetry. |
Congenital/developmental lesions | ||
Congenital absence of the internal carotid artery | Demonstrating an absence of the bony carotid canal with skull base CT will confirm the diagnosis of agenesis. Similarly, demonstrating the presence of a diminutive carotid canal with CT will permit one to differentiate hypoplasia of the internal carotid artery from acquired conditions resulting in a small-caliber internal carotid artery (e.g., chronic dissection, fibro-muscular dysplasia, and severe atherosclerosis). | Agenesis, aplasia, and hypoplasia of the internal carotid artery are rare congenital anomalies (in < 0.01% of the population). Congenital absence may be unilateral (with left-sided predominance) or bilateral. Collateral blood flow may allow these patients to remain asymptomatic. |
Inflammatory/infectious conditions | ||
Reactive lymphadenopathy | In reactive adenopathy, the suprahyoid deep cervical lymph nodes (level II) may by definition enlarge to a maximum diameter of 1.5 cm but maintain their normal oval shape, isodensity to muscle, and their homogeneous internal architecture with variable, usually mild enhancement. Enhancing linear markings within node may be seen. Postinflammatory fatty infiltration of nodes appears as low-density nodal hilus, mimicking necrosis, in a node with pronounced lima-bean shape. | The internal jugular nodal chain is closely associated, but not in the carotid space. Reactive hyperplasia is a nonspecific lymph node reaction, current or prior to any inflammation in its draining area. Can occur at any age, but most common in the pediatric age group. |
Viral lymphadenitis | Viral adenitis may present as bilateral diffuse lymph node enlargement without necrosis, similar in appearance to lymphoma and sarcoidosis. | Nonspecific imaging finding that may be observed in a variety of viral infections, such as adenovirus, rhinovirus, enterovirus, measles, mumps, rubella, varicella zoster, herpes simplex virus, Epstein–Barr virus, and cytomegalo-virus. However, in the presence of parotid lymphoepithelial cysts and hyperplastic adenoids, HIV infection should be strongly suggested. |
Suppurative lymphadenitis | In suppurative adenopathy, the involved nodes are enlarged, ovoid to round, with poorly defined margins and surrounding inflammatory changes. Contrast-enhanced CT images show thick enhancing nodal walls with central hypodensity, similar to metastatic adenopathy with necrotic center. Extracapsular spread of infection from the suppurative lymph nodes may result in abscess formation. | Common in pediatric age group with acute onset of tender neck mass and fever. Organisms have predilection for specific ages (infants: Staphyloccoccus aureus, group B, Streptococcus, Kawasaki disease; children 1–4 y: S. aureus, group A, Streptococcus, atypical mycobacteria; children 5–15 y: anaerobic bacteria, toxoplasmosis, cat scratch disease, tularemia; immunocom-promised adults: histoplasmosis, coccidioidomycosis, Cryptococcus, Pneumocystis carinii, and toxoplasmosis). |
Carotid space infection | Cellulitis may present as a soft tissue mass with obliteration of adjacent fat planes. It is often ill defined, enhancing, and extending along fascial planes and into subcutaneous tissues beneath thickened skin. Abscesses often appear as a poorly marginated soft tissue mass in the expanded carotid space with single or multiloculated low-density center, with or without gas collections, and usually thick abscess wall. Contrast-enhanced CT images show a thick, irregular peripheral rim enhancement and enhancement of the inflamed adjacent soft tissues. | Infection of the carotid space often evolves from a suppurative adenitis of internal jugular chain lymph nodes draining areas of submandibular, tonsillar, or pharyngeal infection. It may also result from extension of cellulitis or abscess from adjacent spaces or after direct penetrating trauma. Carotid space infection may result in a septic thrombophlebitis of the internal jugular vein or in an internal carotid artery erosion, thrombosis, or pseudoaneurysm formation. |
Vascular lesions | ||
Jugular vein thrombophlebitis or thrombosis | Jugular vein thrombophlebitis: Characterized by enlargement of the internal jugular vein by intraluminal hyperdense acute thrombus; also, increased density in fat and loss of soft tissue planes surrounding the thrombus-filled vein from edema/cellulitis. After intravenous (IV) administration of contrast material, the thickened vessel wall may enhance intensely, contrary to the nonenhancing luminal thrombus. Edema fluid may be present in the retropharyngeal space. Jugular vein thrombosis: The vein is dilated, with low-attenuation intraluminal content and enhancement of the wall, without adjacent inflammation. Collateral veins bypassing the thrombosed jugular vein may be seen. | In the acute-subacute thrombophlebitis phase (< 10 d after acute event), the clinical findings are most commonly those of a swollen, hot, tender, ill-defined, nonspecific neck mass with fever. In the chronic jugular vein thrombosis phase (> 10 d after acute event), patients present with a tender, vague, deep, and nonspecific neck mass, in comparison with a palpable cord encountered when the external vein is thrombosed. A history of central venous catheter placement, pacemaker insertion, previous neck surgery, local malignancy, infective cervical adenopathy, deep space infection, polycythemia, or drug abuse is often available. The hallmarks of the Lemierre syndrome are (1) septic jugular vein thrombosis after a primary oropharyngeal infection and (2) metastatic infection. |
Venous aneurysm | Fusiform or saccular dilation of a cervical vein with attenuation values similar to the brachiocephalic vein before and after contrast administration that enlarges on Valsalva maneuver. | Aneurysmal dilations in cervical veins are rare due to low pressure in the venous system. They are observed in any cervical vein, most frequently in the internal and external jugular veins. Congenitally elastic layers and muscle cells are insufficient or even absent in the wall of venous aneurysms. May appear as a soft, compressible mass in the neck. |
Internal carotid artery aneurysm | Fusiform, eccentric, or saccular dilation of the internal carotid artery with attenuation values similar to the aorta before and after contrast administration, often associated with parietal thrombus. Curvilinear calcification of the dilated vascular wall is common. | Extracranial carotid aneurysms are uncommon. Atherosclerosis, fibromuscular dysplasia, trauma, spontaneous dissection, and infection are causes of extracranial aneurysms. Most arise from the bifurcation or proximal internal carotid artery and can rupture, thrombose, or cause distal emboli. Ischemic symptoms and mass effect are the typical clinical presentation. |
Carotid artery dissection | Internal carotid artery dissection typically spares the carotid bulb and terminates near the skull base. In the case of a subintimally located dissection, the mural hematoma will protrude the intima into the vessel lumen and produce a variable degree of stenosis up to occlusion. Dissections that occlude the internal carotid artery may terminate in a rat tail–shaped tapered occlusion. However, if the dissection occurs in the subadventitial layer, vessel wall thickening and considerable wall expansion into the carotid space may occur without causing relevant vessel narrowing. Focal outpouchings of the enhancing arterial lumen (pseudoaneurysms), without associated thrombus, may be seen on CT or CTA. A discrete intimal flap and patent double lumens are rarely seen findings. | Carotid artery dissections result from intimal injury, laceration of the arterial wall, or spontaneous hemorrhage of the vasa vasorum, causing subintimal or intramural hematoma. Nontraumatic dissection can be in association with an underlying vasculopathy (e.g., fibromuscular dysplasia), hypertension, migraine headaches, vigorous physical activity, pharyngeal infections, sympathomimetic drugs, and oral contraceptives. Dissections may cause headache, neck or suboccipital pain, cerebral ischemia, and infarction (from either flow reduction or thromboembolic complications). Symptom onset may be delayed. Some patients develop postganglionic Horner syndrome or lower cranial nerve palsies. |
Carotid artery pseudoaneurysm | Pseudoaneurysm is seen as a carotid space mass. Axial contrast-enhanced CT scans demonstrate focal outpouchings of the enhancing arterial lumen, often in an orientation parallel to the vessel with a double lumen sign. The false lumen of a large pseudoaneurysm may compress the true lumen of the displaced parent carotid artery. Enhancement may be irregular with associated intraluminal thrombus. Wall calcification occurs if chronic. | Rupture of all three arterial layers accompanied by an organized hematoma that then cavitates and communicates with the true vessel lumen results in a pseudoaneurysm. Pseudoaneurysms do not contain normal arterial wall components. The development of a pseudoaneurysm of the extracranial carotid arteries is rare. Patients with advanced neck cancer, radiation therapy, and iatrogenic injury during surgery, after blunt or penetrating trauma, are at risk. Symptoms vary from an asymptomatic neck mass to sudden rupture with devastating hemorrhage and death. |
Carotid artery rupture | The soft tissue hematoma appears as a well to poorly defined, often inhomogeneous mass lesion that may displace the adjacent structures. CT densities range from 80 HU (acute) to 20 HU (chronic). Rarely, a fluid–fluid level is evident, caused by the setting of cellular elements within the hematoma. Contrast-enhanced CT images may show active extravasation of contrast material. CTA can be very effective in identifying the site or source of bleeding. | Rupture of the extracranial carotid artery or its branches is a rare but serious complication accompanied by life-threatening bleeding, pseudoaneurysm, or arteriovenous fistula. This “carotid blowout” syndrome is usually a result of radiation therapy for head and neck cancer, more extensive surgery, wound breakdown, infection, and tumor recurrence; may occur in patients with retropharyngeal abscess, parapharyngeal abscess, and necrotic fasciitis; is secondary to a pseudoaneurysm or as a result of trauma. The site of the hemorrhage is the internal carotid artery in 62% of cases, followed by the external carotid artery (25%) and common carotid artery (13%). Patients with an impending carotid rupture present with a sentinel hemorrhage with profuse but self-limited bleeding. Acute carotid blowout refers to acute uncontrolled hemorrhage. |
Benign neoplasms | ||
Glomus vagale paraganglioma | Sharply defined, ovoid mass, isodense to adjacent muscles, and surrounded by fat planes. The tumor is intensely and homogeneously enhancing after IV bolus injection of contrast material. On dynamic CT scanning, glomus tumors have a vascular curve. At suprahyoid levels, the carotid space paraganglioma typically displaces the internal carotid artery anteromedially (without widening of the carotid bifurcation), the internal jugular vein posterolaterally, the parapharyngeal space and the styloid muscles anteriorly, the styloid process anterolaterally, and the posterior belly of the digastric muscle laterally. Upper cervical paraganglioma may show vertebral destruction. When the tumor extends to the level of the jugular foramen, permeative bone changes may be noted. | Glomus vagale paragangliomas (2.5% of all paragangliomas) are usually centered in the nasopharyngeal carotid space, 2 cm below the skull base (nodose ganglion of the vagus nerve) but can arise from anywhere along the course of the vagus nerve. Occurs in sporadic and familial forms. Five percent are multicentric in the nonfamilial group and may be multiple in 30% of patients with a positive family history of paraganglioma. They can be associated with medullary carcinoma of the thyroid, other visceral neoplasms, in familial multiple endocrine neoplasia syndromes (especially multiple endocrine neoplasia [MEN] type 1), neurofibromatosis, and multiple mucocutaneous neuromas. The majority of patients are younger than 40 y (slight female predominance). Glomus vagale tumors may present as an asymptomatic, slowly enlarging, nontender, pulsatile neck mass or posterolateral pharyngeal mass but more commonly present with symptoms of vagus nerve dysfunction (vocal cord paralysis) or with symptoms from involvement of the hypoglossal or glossopharyngeal nerves. Glomus vagale tumor is rarely hormonally active. Malignant paragangliomas are very rare. |
Schwannoma | Usually well-circumscribed, ovoid to fusiform, homogeneous soft tissue mass, isodense or, rarely, hypodense to adjacent muscles with variable, often intense contrast enhancement. On dynamic CT scanning, schwannomas show either a rapid or a gradual enhancement pattern. Large tumors may undergo cystic degeneration and therefore present with central unenhancing and peripheral enhancing areas (target appearance). Calcifications are exceptional. In suprahyoid neck, carotid space schwannomas, surrounded by fat planes, typically grow in a craniocaudal direction posterior to the internal carotid artery and the internal jugular vein, tend to separate the vessels, and displace the internal carotid artery anteromedially, the internal jugular vein posterolaterally, the parapharyngeal space and styloid muscles anteriorly, the styloid process anterolaterally, and the posterior belly of the digastric muscle laterally. If the jugular foramen is involved, an expanded jugular foramen with sharp, sclerotic margins is characteristic. | Schwannomas are rare (5% of all benign soft tissue tumors). Schwannomas of the suprahyoid carotid space arise from CN IX to XII, the cervical nerve roots, or the sympathetic chain. Mean age at onset is 18 to 63 y (male predominance). Schwannomas are usually solitary and present as a slowly enlarging, painless anterolateral neck mass and/or posterolateral pharyngeal wall mass. Ipsilateral vocal cord paralysis represents the most common symptom of a vagus nerve schwannoma. May be multiple with neurofibromatosis type 2. Malignant transformation is exceedingly rare. |
Neurofibroma | Solitary neurofibroma: Fusiform, ovoid or tubular, sharply circumscribed mass, isodense to cervical cord, smooth and surrounded by fat planes. Differs from schwannoma by an overall lower density that may approach water and absence of contrast enhancement. In suprahyoid neck, carotid space solitary neurofibromas grow posterior to the internal carotid artery and the internal jugular vein, tend to separate the vessels, and displace the internal and external carotid artery anteromedially, the internal jugular vein posterolater-ally, the parapharyngeal space and styloid muscles anteriorly, the styloid process anterolaterally, and the posterior belly of the digastric muscle laterally. Plexiform neurofibroma: Usually large, diffuse, ill-defined, lobulated, multinodular, low-density mass involving multiple cervical compartments (transspatial), including the carotid space. A typical sign is the target sign with central punctate enhancement surrounded by peripheral low attenuation within multiple tumor nodules. Neurofibromas observed in von Recklinghausen disease characteristically occur at multiple sites and are often confluent, multiple, or plexiform. | About 50% of all neurofibroma cases are sporadic, ~50% are associated with neurofibromatosis type 1. Neurofibromas of the suprahyoid carotid space arise from the sympathetic chain, vagus nerve, or hypoglossal nerve. They can occur at any age, with the average 35 y (female predominance). These neoplasms grow slowly and are painless. Large masses can result in complex lower cranial nerve palsies. Two to 6% of lesions in neurofibromatosis type 1 may undergo malignant transformation. |
Meningioma | The jugular foramen meningioma is a dural-based, well-circumscribed mass, isodense or hyperdense, or even calcified. Compared with schwannoma it enhances strongly and may demonstrate dural origin. The adjacent cortex shows sclerosis, remodeling, or erosion. As the tumor extends out of the foramen, it may cause smooth enlargement of the foramen and assume a dumbbell shape with intra- and larger extracranial components. The internal carotid artery is pushed anteriorly by the emerging carotid space meningioma. | Extracranial extension of a dumbbell-type jugular foramen meningioma, though rare, may descend into the carotid space and present as a slow-growing carotid space mass with gradual symptom progression of complex lower cranial neuropathy (female predominance; age 40–60 y). |
Malignant neoplasms | ||
Nodal metastases | Lymph nodes in the upper internal jugular chain are considered abnormal when > 1.5 cm in diameter or when there is evidence of central hypodensity suggesting necrosis. | The lymphatics of the nasopharynx, oral cavity, oropharynx, supraglottic larynx, and pyriform sinus may drain to the upper internal jugular nodes. Level II nodes are the most commonly metastatic involved with regard to all head and neck squamous cell carcinoma. |
Lymphoma | Hodgkin and non–Hodgkin lymphomas can present with multiple or single nodal enlargement as isolated symptom or as part of a more advanced stage. In general, there is a homogeneous enhancement, although rim enhancement or spotty or dentritic enhancement on CT is sometimes seen. | The upper internal jugular lymph node chain may also be involved by malignant lymphoma. |
Contiguous tumor extension | Soft tissue mass within the carotid space, obliterating the normal fat planes and enhancing vessels. | Squamous cell carcinoma may extend outside the pharyngeal mucosal space. The most common malignant processes involving the suprahyoid carotid space are direct invasion by nasopharyngeal or tonsillar carcinoma. |







Disease | CT Findings | Comments |
Pseudomass | ||
Tortuous carotid artery | One or both internal carotid arteries may have a retropharyngeal course and are imposing as round enhancing structure in the widened retropharyngeal space (in fact, the artery is not contained within the retropharyngeal space; instead, it bows the alar fascia medially and projects into the retropharyngeal space). Bilateral ectatic internal carotid arteries migrating medially to touch in the midline of the retropharyngeal space are called “kissing carotids.” If the carotid artery folds sharply on itself, the appearance of an enhancing carotid space mass may be suggested. Sectional imaging, reconstructions, and CTA easily define the vascular nature of the tubular, tortuous, sometimes dilated carotid artery. | A tortuous internal carotid artery can manifest as a pulsatile mass in the carotid triangle at physical examination. It can also present as a submucosal pulsatile mass displacing the pharyngeal posterior wall. Increasing incidence of medial loop, coiling, and kinking are seen with increasing age. |
Retropharyngeal edema | Uniform low-density fluid collection in the retropharyngeal space without significant mass effect, without wall enhancement, or surrounding cellulitis. Sharp demarcation from pharynx and prevertebral muscles. | Accumulation of noninfected fluid in the retropharyngeal space, seen with superior vena cava syndrome, internal jugular vein thrombosis, lymphatic obstruction secondary to lower neck or mediastinal tumor, trauma, neck surgery, or radiation. It is also seen in patients with acute calcific prevertebral tendinitis (longus colli tendinitis) or as a result of infections of the pharynx or vertebral column. Retropharyngeal fluid itself is asymptomatic. |
Congenital/developmental lesions | ||
Infantile hemangioma (capillary hemangioma) | Solitary or multifocal, lobular soft tissue mass, homogeneous and isodense with muscle. Contrast enhancement is uniform and intense. No calcifications. Infantile hemangiomas are usually infiltrative lesions that do not respect the fascial boundaries and may extend into the retropharyngeal space as part of multiple space involvement (transspatial diseases). Size, enhancement, and vascularity of tumor regress in involutional phase with internal, low-density fat. | True capillary hemangiomas are neoplastic conditions; they typically display a rapid proliferation phase during the first year of life followed by an involution phase with fatty replacement. They have a female predilection. Infantile hemangiomas are high-flow lesions during their proliferative phase, low-flow lesions in their involutional phase. Sixty percent of infantile hemangiomas occur in the head and neck, with superficial strawberry-colored lesions and facial swelling and/or deep lesions, often in parotid, masticator, and buccal spaces. Retropharyngeal, sublingual, and submandibular spaces and oral mucosa are other common locations. |
Venous vascular malformation (cavernous hemangioma) | Lobulated or poorly marginated soft tissue mass, isodense to muscle, with rounded calcifications (phleboliths). May be superficial or deep, localized or diffuse, solitary or multifocal, circumscribed or transspatial, with or without satellite lesions. Contrast enhancement is patchy or homogeneous and intense. Bony deformity of the adjacent mandible and fat hypertrophy in adjacent soft tissues may occur. | As opposed to infantile hemangiomas, vascular malformations are not tumors but true congenital low-flow vascular anomalies, have an equal gender incidence, may not become clinically apparent until late infancy or childhood, virtually always grow in size with the patient during childhood, and do not involute spontaneously. Vascular malformations are further subdivided into capillary, venous, arterial, lymphatic, and combined malformations. Venous vascular malformations, usually present in children and young adults, are the most common vascular malformations of the head and neck. The buccal region and dorsal neck are the most common locations. Masticator space, sublingual space, tongue, lips, and orbit are other common locations. Frequently, venous malformations do not respect fascial boundaries and involve more than one deep fascial space (transspatial disease). They are soft and compressible. Lesion may increase in size with Valsalva maneuver, bending over, or crying. Swelling and enlargement can occur following trauma or hormonal changes (e.g., pregnancy). Pain is common and often caused by thrombosis. |
Lymphatic malformation (lymphangioma, cystic hygroma) | Uni- or multiloculated, nonenhancing, fluid-filled mass with imperceptible wall, which tends to invaginate between normal structures. Often found in multiple contiguous cervical spaces (transspatial) with secondary extension into the retropharyngeal space. Large retropharyngeal lymphangioma may cause mass effect on pediatric airway. Rapid enlargement of the lesion, areas of high attenuation values, and fluid–fluid levels suggest prior hemorrhage. Lymphatic and venous malformation may coexist. | Lymphatic malformations represent a spectrum of congenital low-flow vascular malformations, differentiated by size of dilated lymphatic channels. May be sporadic or part of congenital syndromes (Turner, Noonan, and fetal alcohol syndromes). Macrocystic lymphatic malformation is the most common subtype. Sixty-five percent are present at birth. Ninety percent are clinically apparent by 3 y of age; the remaining 10% present in young adults. In the suprahyoid neck, the masticator and submandibular spaces are the most common locations. In the oral cavity, lymphatic malformations can occur in the tongue, floor of the mouth, cheek, and lips. The anterior tongue is the most common location for oral cavity lymphangiomas, commonly presenting as an enlarged tongue. |
Inflammatory/infectious conditions | ||
|
| Infection of the retropharyngeal space is most common in children age 6 y or younger in whom pharyngitis or infection of the faucial tonsils of the oropharyngeal mucosal space or the adenoids of the nasopharyngeal mucosal space, most often with streptococci or staphylococci, spread to the retropharyngeal lymph node chains. Infection of the retropharyngeal space is uncommon in adults, in whom it is most often due to accidental (foreign body, fish bones, or gunshots) or iatrogenic (endoscopy, intubation, or assisted ventilation) pharyngeal perforation. Typically, retropharyngeal space infections progress in four successive phases and can be stopped at any stage by appropriate treatment: |
Reactive lymphadenopathy | In reactive lymphadenopathy, the suprahyoid retropharyngeal lymph nodes are enlarged (> 8 mm) but maintain their normal oval shape, isodensity to muscle, and homogeneous internal architecture with variable, usually mild enhancement. Enhancing linear markings within the node may be seen. | 1. Phase: Reactive hyperplasia is a nonspecific lymph node reaction current or prior to any inflammation in its draining area. Reactive lymphadenopathy also represents the first response of the retropharyngeal lymph nodes to the spread of infection. |
Suppurative lymphadenitis | In suppurative lymphadenitis, the involved retropharyngeal nodes are enlarged, ovoid to round, with poorly defined margins and surrounding inflammatory changes. Contrast-enhanced CT images show thick, enhancing nodal walls with central hypodensity, similar to metastatic adenopathy with necrotic center. | 2. Phase: The infected nodes suppurate with consequent development of an intranodal abscess. |
Cellulitis | Cellulitis is seen as horizontal, rectangle, or oval widening in the posterior midline of the retropharyngeal space (nonnodal pattern of retropharyngeal space involvement: mass displaces carotid spaces laterally, pharyngeal mucosal space anteriorly, while flattening prevertebral muscles posteriorly) with poorly defined areas of low density and an amorphous enhancement following contrast media injection. The process may extend into the adjacent spaces and inferiorly into the mediastinum. | 3. Phase: Early spread of the organism outside an infected suppurative lymph node may result in retropharyngeal cellulitis, causing the tissues to swell without focal fluid collections. |
Abscess | Tense fluid collection (the attenuation value of the liquefied content usually exceeds 25 HU), distending the retropharyngeal space in a nonnodal pattern of retropharyngeal space involvement and producing pharyngeal airway narrowing. Presence of gas within is virtually diagnostic. Thick, irregular enhancing wall suggests mature abscess. Adjacent cellulitis/phlegmon may obscure the prevertebral muscles and pharyngeal mucosal space structures. Complications may result from spread to adjacent spaces (mediastinitis with 50% mortality, jugular vein thrombosis or thrombophlebitis, narrowing of the internal carotid artery [ICA] caliber, and ICA pseudoaneurysm and rupture). | 4. Phase: Extracapsular spread of infection from a suppurative lymph node rupture results in abscess formation. Can also result from ventral spread of diskitis/osteomyelitis and prevertebral infection or from accidental or iatrogenic pharyngeal perforation. Most patients are children (< 6 y). Increasing frequency in adult population because of diabetes, HIV, alcoholism, and malignancy. Common signs: septic male patient with neck pain and sore throat. |
Benign neoplasms | ||
Lipoma | Retropharyngeal, well-defined, nonenhancing fat density mass (–65 to –125 HU), homogeneous without any internal soft tissue stranding. Benign infiltrating lipoma may involve multiple contiguous neck spaces. | Lipomas of the retropharyngeal space are rare and do not cause symptoms until they reach a large size and have a significant mass effect on the pharynx (obstructive sleep apnea). Such fatty tumors also carry the rare possibility of being liposarcomas (inhomogeneous mass with soft tissue and fatty components and contrast enhancement), which further warrants their excision. |
Malignant neoplasms | ||
Nodal metastases | Uni- or bilateral round retropharyngeal mass with a nodal pattern of suprahyoid retropharyngeal space involvement; the center of the lesion is anterior to the prevertebral musculature, posteromedial to the parapharyngeal space, and medial to the carotid space. Displacement of the parapharyngeal space is anterolateral. The mass flattens and remains anterior to the prevertebral musculature. Necrosis appears as central low density with a variably thick, irregular enhancing wall. Ill-defined margins and stranding of surrounding fat are features of extracapsular spread. May have significant mass effect on pharynx. | Metastatic involvement of lateral and medial retropharyngeal lymph nodes is most commonly seen with a nasopharyngeal primary squamous cell carcinoma, but also with an oropharyngeal, nasal cavity, and hypopharynx carcinoma. In addition, the retropharyngeal lymph nodes may be involved by thyroid carcinoma, malignant melanoma, and breast carcinoma. |
Lymphoma | Involved nodes initially appear enlarged, homogeneous, and mildly enhancing. Central hypodense necrosis is uncommon. Later extranodal progression may cause the lymphomatous tissue to fill the entire retropharyngeal space. | The retropharyngeal lymph node chain may be involved by non–Hodgkin lymphoma, Hodgkin disease, and chronic lymphocytic leukemia, either as an initial site or as part of a multiple chain and/or extranodal lymphatic or extranodal extralymphatic involvement. |
Contiguous tumor extension | At the level of the nasopharynx, an ill-defined soft tissue mass of the posterior wall may be seen with extrapharyngeal tumor spread into the soft tissues of the retropharyngeal space in a nonnodal pattern of retropharyngeal space involvement. Once inside, the retropharyngeal tumor can move in a cephalocaudal direction. Associated malignant lymphadenopathy in the suprahyoid retropharyngeal space may be present. | Squamous cell carcinoma may extend outside the pharyngeal mucosal space. The most common malignant processes involving the suprahyoid retropharyngeal space are direct contiguous extension from nasopharyngeal, posterior oropharyngeal wall, or tonsillar carcinoma. Nasopharyngeal lymphoma, rhabdomyosarcomas, or minor salivary gland malignancies may also involve the retropharyngeal space by direct extension from the nasopharynx (visceral space) or involvement of the retropharyngeal lymph nodes. |
Trauma | ||
Emphysema | Interstitial radiolucent gaseous collections in the retropharyngeal space and in several other spaces, including subcutaneous tissues. Extensive soft tissue emphysema may extend downward, giving rise to pneumomediastinum. | Gas in the retropharyngeal space secondary to laryngeal trauma and accidental (foreign body, fish bones, and gunshots) or iatrogenic pharyngeal perforation (endoscopy, intubation, and assisted ventilation) is well known. |
Hematoma | Tense fluid collection distending the retropharyngeal space in a nonnodal pattern of retropharyngeal space involvement producing pharyngeal airway narrowing. In the acute stage, the hematoma may be hyperdense and homogeneous. In the subacute stage, the hematoma may be either isodense or slightly hypodense, with patchy or inhomogeneous appearance. When completely liquefied, the hematoma again has a homogeneous density that is lower than muscle and may be surrounded by a pseudocapsule, which enhances after contrast administration. | Hematomas in the retropharyngeal space are unusual and are most often related to blunt head and neck trauma. Nontraumatic causes of retropharyngeal hematoma include anticoagulant therapy and complications of aneurysms, tumors, and infection. Life-threatening airway obstruction can result from retropharyngeal hematomas. |







Disease | CT Findings | Comments |
Pseudomass | ||
Levator scapulae muscle hypertrophy | Altered contour of the neck with homogeneous enlargement of levator scapulae muscle and denervated atrophic trapezius muscle. Absent ipsilateral internal jugular vein and sternocleidomastoid muscle from radical neck dissection, or an ipsilateral jugular foramen mass are findings of underlying cause. | Asymmetry of the levator scapulae muscles is an unusual cause of a palpable posterior triangle mass. Damage to the spinal accessory nerve secondary to radical neck dissection or, less commonly, an ipsilateral jugular foramen mass (paraganglioma, schwannoma, meningioma, or metastasis) leads to denervation atrophy of the trapezius muscle with compensatory hypertrophy of the levator scapulae muscle. In case of levator scapulae muscle atrophy secondary to cervical spondylosis with spinal nerve compression of the C3, C4, and C5 roots, the normal-sized contralateral levator scapulae muscle may present as a palpable mass. |
Levator claviculae muscle | Uni- or bilaterally, the levator claviculae muscles arise from the anterior portion of the transverse processes of the upper cervical vertebrae (most likely above the C3 level), then head inferiorly and laterally, coursing lateral to the scalene muscles, anterior to the levator scapulae muscle, and medial to the sternocleidomastoid muscle, and insert in the lateral third of the clavicle, blending with the trapezius. | A normal variant not to be mistaken for an abnormality (lymphadenopathy or unenhanced vessel). |
Vertebral body osteophyte | The classic sign of spondylosis is osteophytosis. Osteophytes are bony spurs that originate on the anterolateral aspect of the vertebral bodies a few millimeters from the discovertebral junction (at the site of attachment of the peripheral fibers of the annulus fibrosus). At the beginning, they extend in a horizontal direction; in the more advanced phase, they become hooked and grow vertically. Sometimes osteophytes develop on both sites of a disc space and grow until they fuse together to form a “bridge” osteophyte. | Spondylosis deformans of the cervical spine is the most typical consequence of age- or load-related degeneration of the vertebral body. It is found in 60% of women and 80% of men after the age of 50. C5–C6 is the most common level, followed by C6–C7, then C4–C5. Only when degenerative alterations are severe and symptomatic (e.g., cervical osteophytic dysphagia) should they be considered pathologic. Osteophytes can be distinguished from syndesmophytes of ankylosing spondylitis, paravertebral ossification of psoriasis and reactive arthritis, and bulky anterior-flowing ossification of the anterior longitudinal ligament of diffuse idiopathic skeletal hyperostosis. |
Facet degenerative arthropathy | Osseous facet overgrowth (“mushroom cap” facet appearance) and bone excrescences impinging on neural foramina and the spinal canal in conjunction with articular joint space narrowing with sclerosis and intra-articular gas (vacuum phenomenon). Enhancing inflammatory soft tissue changes surrounding the facet joint are common. Frequently seen in conjunction with spondylosis deformans. | Hypertrophic degenerative facet may be perceived as a cervical mass on physical examination. Arthropathy most common in middle/lower cervical spine. Frequently seen in the elderly population. No gender preference. |
Congenital/developmental lesions | ||
Venous vascular malformation (cavernous hemangioma) | Lobulated soft tissue mass, isodense to muscle, containing rounded calcifications (phleboliths). Contrast enhancement may be patchy and delayed or homogeneous and intense. Fat hypertrophy in adjacent soft tissues may be present. | Most common vascular malformation of head and neck, commonly in buccal region. Dorsal neck is another common location. These are usually infiltrative transspatial lesions and may extend into the perivertebral space as part of multiple space involvement. Present clinically in children, adolescents, or young adults. |
Lymphatic malformation (lymphangioma, cystic hygroma) | Uni- or multiloculated, nonenhancing, fluid-filled mass with imperceptible wall, which tends to invaginate between normal structures. Often found in multiple contiguous cervical spaces (transspatial). Rapid enlargement of the lesion, areas of high attenuation values, and fluid–fluid levels suggest prior hemorrhage. Lymphatic and venous malformation may coexist. | Lymphatic malformations represent a spectrum of congenital low-flow vascular malformations, differentiated by size of dilated lymphatic channels. May be sporadic or part of congenital syndromes (Turner, Noonan, and fetal alcohol syndrome). Macrocystic lymphatic malformation is the most common subtype; 65% are present at birth. Ninety percent are clinically apparent by 3 y of age; the remaining 10% present in young adults. In the suprahyoid neck, the masticator and submandibular spaces are the most common locations. In the oral cavity, lymphatic malformations can occur in the tongue, floor of the mouth, cheek, and lips. The anterior tongue is the most common location for oral cavity lymphangiomas, commonly presenting as an enlarged tongue. |
Inflammatory/infectious conditions | ||
Vertebral body osteomyelitis | Infectious spondylitis of the cervical spine is discocentric and extends from one vertebral body to adjacent vertebra across a disc; may reveal involvement at a single level or at two contiguous levels. The typical CT findings of pyogenic vertebral osteomyelitis include irregularity and loss of vertebral end plate cortex of two adjacent vertebrae; Swiss cheese–like, diffuse lytic vertebral body destruction with extensive bone sequestration (particularly in the subchondral bone region); and destruction of the intervertebral disc with loss of the adjoining disc space height. The paraspinal soft tissue component with soft tissue swelling, cellulitis with diffusely enhancing soft tissue edema, and abscess formation tend to involve the entire prevertebral space. Gas within both bone and adjacent soft tissue is a reliable indicator of infection. Spinal cord compression may occur because of intraspinal extension. The prevertebral space infection may secondarily involve the retropharyngeal space and carotid space. Osteomyelitis involving the posterior elements of the spine with an inflammatory mass in the paraspinal portion of the perivertebral space is rare. The classic CT findings of tuberculous spondylitis are large, frequently calcified paraspinal soft tissue masses with thick, irregular rim enhancement and focal lytic anterior vertebral body destruction, associated with marginal sclerosis. Isolated posterior element involvement is possible. | The cervical spine is an uncommon site for osteomyelitis. C5 and C6 levels are most frequently affected (6.5% of all spinal segments involved). Mycobacterium tuberculosis is the most common causative agent worldwide. S. aureus is the most common pathogen in the United States. Brucella, Pseudomonas, Serratia, and Candida are common organisms in long-standing IV drug addicts and immunocompromised patients. Vertebral osteomyelitis is usually caused by hematogenous spread to the vertebral body, whereas infection of the intervertebral disc is due to osteomyelitis of the adjacent vertebral body that secondarily invades the disc or to direct contamination of the disc during surgical spine procedures or penetrating trauma. Adults are more often affected. Diabetics, pediatric and elderly patients, IV drug addicts, and patients with urinary tract infection, pneumonia, or skin infection are at risk. |
Soft tissue infection | Phlegmon of the perivertebral space may present as focal or diffuse muscle enlargement and obliteration of soft tissues, fascial planes and skin, due to diffuse soft tissue edema, with enhancement. Often associated with abscess formation: peripherally enhancing, low-density liquefied collection, with or without gas. | Soft tissue infection of the prevertebral and/or paraspinal portion of the perivertebral space from direct extension from adjacent infection (spondylodiscitis and septic facet arthritis), hematogenous spread from distant sites, or transcutaneous direct inoculation of deep tissue. Most common causative agents are S. aureus, Mycobacterium tuberculosis, and E. coli. Fungal infections are rare, more common in immuno-compromised host. Predisposing factors are diabetes mellitus, alcoholism, cirrhosis, chronic renal failure, IV drug abuse, and immunocompromised state. |
Longus colli tendinitis | Focal soft tissue thickening with calcification in the prevertebral area anterior to C1 and C2. It is associated with a retropharyngeal space edema, extending as a uniform low-density fluid collection in the retropharyngeal space down to C5, without significant mass effect, without wall enhancement, or surrounding cellulitis. Sharp demarcation is seen from the pharynx and prevertebral muscles. Calcifications may resolve on imaging follow-up. | Calcific tendinitis of the longus colli muscle is characterized by the deposition of calcium hydroxyapatite into the longus colli tendon. Patients (third to sixth decade) often complain of odynophagy, neck pain, paraspinal muscle spasm, and mild fever for 2 to 7 days. |
Vascular lesions | ||
Vertebral artery dissection | Extracranial vertebral artery dissections are usually located in the extradural segment between the skull base and C2 (V3), less commonly in the foraminal segment (V2). In the case of a subintimal dissection, the mural hematoma will protrude the intima into the vessel lumen and produce a variable degree of stenosis, irregular stenosis, long segment stenosis (“string” sign), multiple focal stenosis (“string of pearls” sign), up to a rat tail–shaped tapered or abrupt vascular occlusion. However, if the dissection occurs in the subadventitial layer, considerable wall expansion may occur without causing relevant vessel narrowing. Although rare, an intimal flap, a double lumen (dissecting aneurysm), or a focal aneurysmal dilation (pseudoaneurysm) may be seen on CT or CTA. A thin rim of contrast enhancement can sometimes be seen surrounding the mural hematoma (possibly resulting from enhancement of the vasa vasorum). Multivessel involvement may occur in up to two thirds of cases. | Dissection of the vertebral artery is an often overlooked cause of stroke in young adults (< 45 y). Vertebral artery dissections are caused by a primary intramural hematoma (due to ruptured vasa vasorum) or by penetration of blood into the arterial wall through a primary intimal tear. Vertebral artery dissections can be traumatic or nontraumatic. Non-traumatic dissection can be either spontaneous or in association with an underlying vasculopathy (e.g., fibromuscular dysplasia or cystic media necrosis), a predisposing connective tissue disorder (Marfan syndrome, Ehlers–Danlos syndrome, osteogenesis imperfecta, or autosomal dominant polycystic kidney disease), hyperhomocysteinemia, hypertension, migraine, vigorous physical activity, pharyngeal infections, sympathomimetic drugs, and oral contraceptives. Dissections may cause headache or neck/suboccipital pain, accompanied or followed by ischemic symptoms, originating in the vertebrobasilary territory (either from flow reduction or thromboembolic complications). Subarachnoid hemorrhage from intracranial extension may occur in 10%. Rarer clinical manifestations include cervical spine cord ischemia and cervical root impairment. |
Benign neoplasms | ||
Schwannoma | Although some may emanate from the neural canal, thereby widening the spinal neural foramen (“dumbbell” lesion), still others may derive from the branches beyond the foramen and present as a well- circumscribed ovoid to fusiform homogeneous soft tissue mass, isodense to cord, with variable, often intense contrast enhancement. Large tumors may undergo cystic degeneration and therefore present with central unenhancing and peripheral enhancing areas. Calcifications are exceptional. | Tumors of neurogenic origin are some of the more frequently seen benign neoplasms of the perivertebral space. They can produce a mass effect in the paraspinal space. This consists mainly of anterior displacement and effacement of the longus muscle at suprahyoid levels and of the anterior scalene muscle at infrahyoid levels. The occurrence of multiple peripheral nerve schwannomas in patients with neurofibromatosis type 2 is characteristic. |
Neurofibroma | Sporadic neurofibromas may present as solitary or multiple ovoid or fusiform heterogeneous low-density masses with well-circumscribed margins. Absence of contrast enhancement is conspicuous. In neurofibromatosis type 1 (NF1), localized neurofibromas have similar imaging characteristics to solitary neurofibromas, but they are often bilateral, multilevel, diffuse, or plexiform and follow the cervical nerve roots with intraforaminal extension, the brachial plexus, the vagus nerves, and peripheral subcutaneous nerve branches. | Ninety percent of neurofibromas occur as sporadic, solitary tumors. Sixteen to 65% of patients with NF1 have neurofibromas (57% single lesions), most commonly in patients between the ages of 20 and 40 y. Sudden painful enlargement of a neurofibroma in NF1 should suggest malignant transformation (3%–5% of patients with NF1 develop malignant peripheral nerve sheath tumors). |
Aggressive fibromatosis (extra-abdominal desmoid fibromatosis, juvenile fibromatosis) | “Malignant-appearing,” poorly marginated inhomogeneously enhancing soft tissue mass with local invasion of muscle, vessels, nerves, and bone. Most common locations are perivertebral space, supraclavicular area, and masticator space. | Benign soft tissue tumor of fibroblastic origin arising from aponeurotic neck structures. Associated abnormality: Gardner syndrome. Seen at all ages (70% younger than 35 y). Significantly more women are affected. Patients present with a firm, painless neck mass. Paresis depends on the site. Postoperative recurrence rate may reach 25%. |
Other benign mesenchymal tumors | Well-defined homogeneous soft tissue mass with usually moderate, uniform contrast enhancement. | Other benign mesenchymal tumors of the perivertebral space are relatively rare and include (myo) fibroblastic tumors, fibrohistiocytic tumors, rhabdomyoma, mesenchymoma, or myxoma. Clinically, these present as a mass of increasing size, associated with discomfort or pain. |
Osteochondroma | Sessile or pedunculated osseous “cauliflower” lesion with marrow and cortical continuity with parent vertebra, usually in the posterior elements of the cervical spine. May compress the spinal cord or nerve roots. | Only 2% to 3% of osteochondromas (sporadic, hereditary multiple exostosis) occur in the spine: cervical (50%, C2 predilection) > thoracic > lumbar > sacrum. Peak age 10 to 30 y; M:F = 3:1. Many spinal osteochondromas are asymptomatic. Complications include deformity, fracture, vascular compromise, neurologic sequelae, mechanical impingement symptoms, and malignant transformation (< 1% solitary lesions; 3%–5% familial osteochondromatosis). |
Aneurysmal bone cyst | Aneurysmal bone cysts arise in the posterior elements of the vertebra as a markedly expansile (“ballooning”) multicystic osteolytic lesion with well-defined scalloped margins and displacement of the paraspinal musculature away from the spine. Fluid–fluid levels (due to hemorrhage and blood product sedimentation) are characteristic. Enhancement is confined to the periphery and septations interposed between the blood-filled spaces. Spinal cord compression may occur because of intraspinal extension. Occasionally, the expansile lesion may affect two or more contiguous vertebrae. Solid aneurysmal bone cyst is a rare variant. The solid component predominates and enhances diffusely. | Primary lesions are usually observed in the first, second, and third decades of life, with slight female predominance; 20% occur in the spine. Can coexist with osteoblastoma, giant cell tumor, or enchondroma. |
Giant cell tumor | Lytic, expansile lesion centered in the vertebral body, with heterogeneous contrast enhancement; may contain fluid attenuation regions due to necrosis or focal aneurysmal bone cyst component. Zone of transition is narrow, margin usually not sclerotic. May have cortical breakthrough with apparent soft tissue extension. | In spine, peak incidence in second and third decades of life, with female preponderance. Can undergo sarcomatous transformation (spontaneously or in response to radiation therapy). Primary malignant giant cell tumors are rare. May be associated with aneurysmal bone cyst. |
Osteoblastoma | Involvement of posterior elements of the vertebra is typical. The expansile, lytic lesion with thin, sclerotic margins may contain partially calcified matrix. | Histologically identical to osteoid osteoma but larger and occurs typically in the spine. May be associated with aneurysmal bone cyst. |
Malignant neoplasms | ||
Vertebral body metastases | Focal, multifocal, or diffuse involvement of the cervical spine with osteolytic, osteoblastic, or mixed-type lesions. The vertebral body is involved before the neural arch. Associated cortical bone destruction and contiguous enhancing soft tissue extension into the perivertebral space are common. Multiple vertebral involvement is seen with intervertebral disc sparing. Bone expansion is virtually limited to lytic metastases from carcinomas of the kidney, thyroid, and lung and osteoblastic metastases from prostatic carcinoma that may mimic Paget disease. Pathologic fracture is common. Spinal cord compression may occur because of intraspinal extension. | Metastatic disease to the vertebral body with extraosseous tumor extension through cortical perfo-rations is the most common malignant lesion of the perivertebral space. Present in 10% to 40% of patients with systemic cancer (primary tumor in adults: lung, breast, prostate, kidney, GI, or unknown primary [15%–25%]; primary tumor in children: hematologic malignancies, neuroblastoma, and Ewing sarcoma). Most common symptoms are spine pain, focal tenderness, soft tissue mass, and neurologic compromise from cord compression. |
Chordoma | Chordoma appears as a destructive vertebral body mass with osseous erosion and expansion, sparing posterior elements. May extend into the disc space and involve adjacent vertebrae. A large associated soft tissue mass of low attenuation with extension into the perivertebral space may be the dominant finding. Mild/moderate contrast enhancement with inhomogeneous necrotic areas is characteristic. Extension along nerve roots and enlargement of neural foramina mimics peripheral nerve sheath tumors. Spinal cord compression may occur because of intraspinal extension. Up to 50% of these tumors may show coarse, amorphous calcified matrix. Anteroinferiorly expanding tumor of the clivus may invade or displace the nasopharyngeal perivertebral space and nasopharynx. | Rare primary malignant tumor of notochord origin. Originates from the sacrum and coccyx region (50%), sphenooccipital region (35%), and spine (15%), especially C2–C5 and lumbar. Cervical spine chordomas occur in 40- to 60-y-old patients (M:F = 2:1) and present with gradual onset of neck pain, numbness, and motor weakness. |
Lymphoma | Involves vertebral body more than neural arch. Multiple vertebrae involvement is common; may cross disc space. Lytic, permeative bone destruction with enhancing poorly defined soft tissue mass involving adjacent structures (epidural, paraspinal muscles) is characteristic. “Ivory” vertebral body is rare. Epidural extension from adjacent vertebral or paraspinous disease is common. | Both in Hodgkin disease and non–Hodgkin lymphoma, bone involvement is usually secondary (hematogenous or invasion from adjacent soft tissues and lymph nodes). Most common presenting symptom is pain, most often in patients between the ages of 40 and 70 y. Slight male predominance. |
Plasmocytoma | Solitary, large, expansile, osteolytic lesion with scalloped, poorly marginated, nonsclerotic margins, associated with soft tissue masses, with mild/moderate homogeneous contrast enhancement. The vertebral body is more frequently involved by plasmocytoma, but the disease may also affect the posterior elements. No tumoral calcifications, but peripherally displaced osseous fragments may be seen. Primary sclerotic form is extremely rare, but sclerosis may occur after proper treatment. May involve intervertebral disc and adjacent vertebrae. Epidural extension and/or variable degrees of pathologic fractures (vertebra plana) may cause spinal cord compression. | Solitary intra- or extramedullary tumor of plasma cells, with no evidence of multiple myeloma elsewhere. Represent only 3% of plasma cell neoplasms. Spine is the most common site. Cervical spine plasmocytomas occur in patients older than 40 y, with male predominance; can be asymptomatic or present with local neck pain, low levels of serum/urine monoclonal proteins, and neurologic compromise from cord compression. Progression to multiple myeloma is common. |
Multiple myeloma | Polyostotic. Common spine presentations are multiple or diffuse lytic lesions, sometimes associated with irregular dense hypertrophy of the remaining vertical trabeculae or diffuse osteopenia. Vertebral destruction and pathologic fractures with variable spinal canal narrowing are common. | Most common primary tumor of bone with multifocal malignant proliferation of monoclonal plasma cells within bone marrow. Occur in 40- to 80-y-old patients (M:F = 3:2), more common in African Americans than Caucasians or Asians. Patients present with bone pain, anemia, hypercalcemia, proteinuria (including Bence–Jones proteins), renal failure, and monoclonal gammopathy (high erythrocyte sedimentation rate, abnormal electrophoresis). |
Malignant mesenchymal tumors | Fairly well to poorly defined, inhomogeneous soft tissue mass with necrotic and hemorrhagic components and considerable contrast enhancement. The center of a mass in the paraspinal portion is within the paraspinal musculature or posterior vertebral body elements, and the mass displaces the paraspinal musculature and posterior cervical space fat away from the spine. Erosion/destruction of adjacent cervical spine is common. | Several different primary neoplasms can arise in the soft tissues of the perivertebral space: malignant fibrous histiocytoma, fibrosarcoma, neurofibrosarcoma, hemangiopericytoma, synovial sarcoma, and rhabdomyosarcoma. The clinical presentation is commonly a painless, slowly growing mass. Acute painful presentations are associated with tumor necrosis or hemorrhage. Metastatic disease within the muscles of the perivertebral space is rare and is seen most frequently with advanced disease. |
Contiguous tumor extension | Imaging findings of obliteration of the retropharyngeal fat stripe, irregular muscle contour, and postcontrast muscle enhancement are suggestive of direct extension from pharyngeal or tonsillar carcinoma with actual prevertebral muscle invasion, but nonspecific (because these findings may also be due to peritu-moral edema without actual muscle invasion). | Late cases of pharyngeal squamous cell carcinoma, particularly those arising in the posterior pharyngeal wall, may rarely invade the perivertebral space. |
Trauma | ||
Hematoma | Soft tissue hematoma appears as prevertebral soft tissue swelling or as a non-neoplastic heterogeneous mass in the paraspinal portion of the perivertebral space, nonenhancing, with adjacent parenchymal hemorrhage and surrounding edema. Unlike hematoma, muscle hemorrhage has little mass effect, with preservation of fascial planes. | Soft tissue hemorrhage can collect as hematoma. Hematomas are a common finding after sport and other injuries associated with cervical spine and muscle injury. Hematomas can be seen in the muscle, in the intermuscular fat planes, or within the subcutaneous tissues. |




Related posts:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree