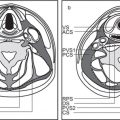
9 Infrahyoid Neck(Table 9.1 – Table 9.4)
Disease | CT Findings | Comments |
Laryngeal lesions | See Table 9.2 | See Table 9.2 |
Thyroid gland lesions | See Table 9.3 | See Table 9.3 |
Congenital/developmental lesions | ||
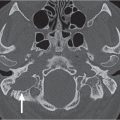
Infrahyoid thyroglossal duct cyst | Nonenhancing, smooth, thin-walled low-density (cystic), round or ovoid midline neck mass, usually between 2 and 4 cm, occasionally septated, located at the level of the hyoid bone (~50% of cases) or below it (~25% of cases), deep to or embedded in the infrahyoid strap muscles (“claw” sign). The more inferior the cyst, the more likely it is to be offthe midline. The wall may thicken and enhance, and the cyst content may develop higher attenuation, if infected. Any associated nodularity or chunky calcification within the cyst suggests associated thyroid carcinoma. Persistent thyroid tissue may occur anywhere along the thyroglossal duct and typically enhances markedly on postcontrast CT. | The thyroglossal duct cyst, found along the course of the embryonic thyroglossal duct, is the most common midline neck mass. Ninety percent of the lesions are diagnosed before the age of 10 y (M < F). Associated abnormalities are thyroid agenesis, ectopia, and pyramidal lobe. |
Cervical thymic cyst | Nonenhancing, smooth, thin-walled, low-density (cystic) mass in the lateral infrahyoid neck, lateral visceral space, or adjacent to the carotid space. The lateral left infrahyoid neck, at the level of thyroid gland, is the most common location. May be unilocular or multilocular. Cyst wall may be nodular (a berrant thymic tissue, lymphoid aggregates, or parathyroid tissue). Larger cyst may present as a dumbbell cervicothoracic mass. | Rare remnant of the thymopharyngeal duct, found from the pyriform sinus to the mediastinum. Most present between 2 and 15 y of age (only 33% present after first decade). Slightly more common in male patients. Often asymptomatic. When large, may be associated with respiratory distress or vocal cord paralysis. |
Fourth branchial anomaly | Nonenhancing, rounded or ovoid, sharply marginated, thin-walled lesion with central fluid density, attached to the thyroid cartilage or with involvement of the left thyroid lobe. When fourth branchial sinus connection with pyri-form sinus is maintained, air may be seen in the sinus tract, cyst, and/or left thyroid lobe area, and infection of the cyst and left thyroid lobe is likely. Thyroiditis and thyroid abscess may obscure the cyst itself. Fourth branchial fistula denotes two openings, one in the low anterior neck and the other in the pyriform sinus apex. | Remnants of the fourth branchial apparatus. Most cases occur anywhere from the left pyriform sinus apex to the thyroid lobe. Rarest of all forms of branchial cleft anomalies (1%–2%). Present as recurrent suppurative thyroiditis or perithyroid abscess in pediatric patients. More common in female patients. |
Inflammatory/infectious conditions | ||
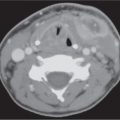
Abscess | Poorly marginated soft tissue mass in the expanded visceral space with single or multiloculated low-density center (the attenuation value of the liquefied content usually exceeds 25 HU), with or without gas collections, and usually thick abscess wall. Contrast-enhanced CT images show a thick, irregular peripheral rim enhancement and enhancement of the inflamed adjacent soft tissues. | Abscess originating in the visceral space is rare and usually secondary to penetrating trauma or superinfection of a hematoma. |
Parathyroid lesions | ||
Parathyroid adenoma | Moderately contrast enhancing, round or oval, well-circumscribed, usually homogeneous soft tissue mass, hypodense to thyroid gland, typically 1 to 3 cm in size, either in the thyroid bed or in an ectopic location. Cystic degeneration and hemorrhage may occur. In a small percentage of cases (2%–5%), multiple adenomas are present. | Normal parathyroid glands (5 × 3 × 1 mm) are usually not seen on CT. Most people (83%) have four parathyroid glands located behind the upper and lower poles of the thyroid gland. Ectopic glands may be located within the thyroid gland (8%) or along the thymopharyngeal duct tract, extending from the angle of the mandible to the lower anterior mediastinum (15%–20%). Primary hyperparathyroidism and hypercalcemia are caused by a solitary adenoma (in 75%–85%, more common in women), multiple adenomas (in 2%–3%), parathyroid hyperplasia (in 10%–15%), or parathyroid carcinoma (in < 1%). |
Parathyroid carcinoma | Parathyroid carcinoma is usually indistinguishable from benign adenoma on sectional imaging. Only the presence of local invasion into the thyroid gland, muscles, or vessels or nodal metastases (in one third of cases) and hematogenous metastases (in 21%–28%) would suggest this diagnosis. | Parathyroid carcinoma causes hyperparathyroidism in 85% to 90% of patients. Men and women are affected equally. |
Parathyroid cyst | Nonenhancing, well-defined, ovoid, usually large (2–12 cm), low-density mass, thin-walled, unilocular, homogeneous, typically found in the tracheoesophageal groove, near the lower pole of the thyroid gland, more common on the left side. The cervicothoracic type may be dumbbell shaped. | Rare entity. Classified as nonfunctioning parathyroid cyst (congenital colloidal cyst, diagnosed in the fourth or fifth decade of life, slightly more common in women) or functioning parathyroid cyst, producing primary hyperparathyroidism (10%; these are pseudocysts with cystic necrosis or degeneration of a parathyroid adenoma, more common in men). Large cysts may compress the adjacent trachea, esophagus, and recurrent laryngeal nerve, causing recurrent laryngeal nerve palsy. |
Hypopharyngeal lesions | ||
Hypopharyngeal carcinoma | Invasive mucosal mass, isodense to muscle, with moderate contrast enhancement, a rising from the pyriform sinus (60%), postcricoid region (25%), or posterior hypopharyngeal wall (15%) with asymmetrical thickening of the pharyngeal walls, tumor-caused deformity of the endopharyngeal contours, and restrictive extensibility with phonation or modified Valsalva maneuver. | More than 90% of tumors of the hypopharynx are squamous cell carcinomas occurring usually in men older than 50 y with a history of tobacco and alcohol abuse. Tumors associated with Plummer–Vinson syndrome typically occur in women. Up to 15% of patients with hypopharyngeal squamous cell carcinoma have second primary tumors. Because of a prolonged asymptomatic preclinical interval, hypopharyngeal cancers are usually in an advanced stage at presentation. Up to 75% of patients have nodal metastases, and 20% to 40% of patients have distant metastases at the time of diagnosis. |
| Tumors arising from the lateral wall of the pyriform sinus tend to spread through the thyrohyoid membrane into the soft tissues of the neck (carotid artery) very early. Tumors originating from the medial wall of the pyriform sinus may infiltrate the larynx by growing anteriorly into the paraglottic space with cord fixation, or they may spread to the postcricoid region and cervical esophagus. Once an advanced pyriform sinus tumor has reached the apex, it may spread into the larynx, invading the arytenoid cartilage, cricoarytenoid joint, and subglottis. Pyriform sinus carcinoma frequently invades the laryngeal framework. Pyriform sinus tumors primarily drain into group II, III, and V lymph nodes. Postcricoid area tumors tend to spread submucosally, either circumferentially or toward the cervical esophagus, with thickening of the postcricoid region and anterior displacement of the arytenoid and cricoid cartilages. Invasion of the cricoid cartilage is common. Growth beyond the posterior cricoarytenoid muscle into the thyroid gland and cervical trachea and perineural infiltration along the branches of the recurrent laryngeal nerve with fixation of the vocal cord are other common features. Postcricoid area tumors tend to metastasize to group III, IV, and VI lymph nodes. Posterior hypopharyngeal wall cancers commonly involve both the hypopharynx and oropharynx with asymmetrical thickening of the posterior pharyngeal wall. Invasion of the retropharyngeal space is common. Rarely, these tumors cause invasion of the prevertebral muscles at presentation. Posterior hypopharyngeal wall cancers have a tendency to involve primarily the retropharyngeal lymph nodes and secondarily the internal jugular chain lymph nodes. | Two to 7% of all hypopharyngeal tumors are a typical forms of squamous cell carcinoma (verrucous carcinoma, spindle cell carcinoma, basaloid cell carcinoma, and undifferentiated carcinoma of the nasopharyngeal type). Carcinoma of the hypopharynx: TNM classification Primary tumor T1: Tumor limited to one subsite* of the hypopharynx and < 2 cm in greatest dimension T2: Tumor invades more than one subsite* of hypopharynx or an adjacent site or measures > 2 cm but < 4 cm in greatest dimension without fixation of hemilarynx T3: Tumor measures > 4 cm in greatest dimension, or with fixation of hemilarynx or extension to esophagus T4a: Tumor invades any of the following: thyroid or cricoid cartilage, hyoid bone, thyroid gland, esophagus, central compartment soft tissue (prelaryngeal strap muscles and subcutaneous fat) T4b: Tumor invades prevertebral fascia, encases carotid artery, or invades mediastinal structures Regional lymph nodes N1: Metastasis in a single ipsilateral lymph node, < 3 cm N2a: Metastasis in a single ipsilateral lymph node between 3 and 6 cm N2b: Metastasis in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension N2c: Metastasis in bilateral or contralateral lymph nodes, none > 6 cm in greatest dimension N3: Metastasis in a lymph node > 6 cm Distant metastasis M1: Distant metastasis * Hypopharyngeal subsites include the paired pyri-form sinuses, the postcricoid area, and the posterior pharyngeal wall. Less than 5% of tumors of the hypopharynx are of nonsquamous cell origin and unrelated to tobacco and alcohol. These include benign tumors (e.g., lipomas, retention cysts of minor salivary glands, leiomyomas, papillomas, adenomas, and angiomatous tumors) and nonsquamous cell malignancies, such as various sarcomas, malignant lymphoma, and malignant minor salivary gland tumors. |
Pharyngocele | Air-filled saccular formation arising either from the lateral side of the pyriform sinus (ostium of junction located between the middle and inferior constrictor muscles) or from the vallecula (ostium of junction located between the superior and middle pharyngeal constrictor muscles). More frequently seen unilateral. Increase in volume with Valsalva maneuver and phonation. | Acquired lateral pharyngeal wall herniation. Presumably, pharyngoceles arise from increased intrapharyngeal pressure, as in chronic coughers, wind instrumentalists, or glass blowers. More common in men (fifth and sixth decade). |
Zenker diverticulum | May appear as a nonenhancing, thin-walled, unilocular oval expansion of the hypopharynx, offthe midline in the posterior visceral space, filled with food, debris, and air, often with an air–fluid level. | Outpouching of mucosa and submucosa at the pharyngoesophageal junction through a weaker area in the posterior pharyngeal wall (Kilian dehiscence). Presenting sometimes with dysphagia or chronic aspiration. |
Esophageal lesions | ||
Esophageal duplication cyst | Cystic-appearing, homogeneous, well- circumscribed, nonenhancing esophageal mass in the visceral space, offthe midline, with mass effect on the trachea and adjacent structures. May extend inferiorly into the upper mediastinum. | Esophageal duplication cysts—a type of gastrointestinal duplication—are rare; 20% occur in the upper third of the esophagus. The cyst lining can be squamous, respiratory, intestinal, or mixed. Gradual enlargement occurs with secretory endothelium. Cysts rarely communicate with the esophagus lumen. Most present in early childhood with dysphagia or respiratory distress. |
Cervical esophageal carcinoma | Often eccentric, poorly marginated, infiltrating, enhancing soft tissue esophageal mass centered in the posterior midline visceral space behind the trachea. Cervical esophageal carcinomas tend to spread in a submucosal fashion to the hypopharynx. Larger lesions are transspatial (retropharyngeal, perivertebral, and carotid space) and may show invasion of thyroid and cricoid cartilage, trachea, and recurrent laryngeal nerve. CT cannot reliably delineate the individual layers of the esophageal wall and therefore cannot distinguish between T1 and T2 lesions. Infiltration of the tumor into the periesophageal fat on CT denotes a T3 tumor. Tumor involving adjacent structures denotes a T4 lesion. Cervical esophageal cancers tend to metastasize to group VI, low level IV or V nodes, and mediastinal lymph nodes. The group VI lymph nodes are involved usually (71%) at the time of diagnosis. | Twenty percent of esophageal squamous cell carcinomas arise in the cervical esophagus (M:F = 4:1; peak range 55–65 y; history of tobacco and alcohol abuse, Plummer–Vinson syndrome, caustic stricture, achalasia, or prior radiation). Frequently detected late with poor prognosis. Diagnosis is established with barium swallow, endoscopy, and biopsy. Cross-sectional imaging is considered complementary and may be performed for staging. Esophageal carcinoma: TNM classification Primary tumor T1: Tumor invades lamina propria, muscularis mucosa T2: Tumor invades muscularis propria T3: Tumor invades adventitia T4: Tumor invades adjacent structures Regional lymph nodes N1: Regional lymph node metastasis Distant metastasis M1a: Cervical nodal metastasis M1b: Distant metastasis Fifteen percent of patients have synchronous or metachronous tumors. |
Juxtavisceral nodal lesions | ||
Nodal metastases from thyroid carcinoma | Lymph node metastases from thyroid carcinoma are round or spherical in shape and may have, irrespective of size, a variable aspect, including homogeneously enhancing nodes, hemorrhagic nodes with high density, central necrotic nodes with peripheral nodal enhancement, or completely cystic nodes (can be filled with high concentration of thyroglobulin that gives low density on CT). Malignant nodal calcification may be seen in metastatic papillary, follicular, and medullary thyroid cancer (42% of cases). An enhancing nodal rim, which is irregular and thick with infiltration to the adjacent fat planes, reflects extracapsular tumor spread. | Juxtavisceral lymph nodes include prelaryngeal, paratracheal, and paraesophageal lymph nodes (level VI nodes). Thyroid carcinoma is the most common neck tumor to involve these nodes exclusively. Papillary carcinoma has the highest incidence of thyroid malignancies for cervical lymph node metastases (50%). |
Lymphoma | In cases of Hodgkin and non–Hodgkin lymphoma, either a single dominant node with scattered surrounding smaller nodes or a multiple nodal disease may be present. Involved lymph nodes range in size from 1 to 10 cm, are round or oval, well circumscribed, often with a thin nodal capsule. Nodal density is equal or less than muscle. In general, there is a homogeneous contrast enhancement, although a thin peripheral rim enhancement or spotty or dendritic enhancement on CT is sometimes seen. Nodal necrosis can occur, albeit less frequently than with metastatic squamous cell carcinoma. Intranodal calcification may be seen after radiation or chemotherapy. Rarely, it may be seen prior to therapy in aggressive high-grade lymphoma. | Both Hodgkin and non–Hodgkin lymphomas involve juxtavisceral lymph nodes, either as an isolated symptom or as part of a more advanced stage. |
Tracheal lesions | ||
Cervical tracheal carcinoma | Soft tissue mass within the outline of the tracheal wall (tracheal cartilage), with narrowing of the tracheal lumen and often with large extratracheal components of tumor (30%–40%). Paratracheal lymph nodes are involved in 30% at presentation. | Primary tracheal malignancy is exceedingly uncommon (0.1%–0.4% of all diagnosed malignancies). Most prevalent histology worldwide is squamous cell carcinoma (60%–90%; men are more often afflicted than women, the usual presenting age in the sixth decade of life, with cough, dyspnea, stridor, hemoptysis, and dysphonia). Other histologies are adenoid cystic carcinoma, carcinoid, mucoepidermoid carcinoma, carcinosarcoma, and chondrosarcoma. Tracheal carcinoma: TNM classification Primary tumor T1: Primary tumor confined to trachea, < 2 cm T2: Primary tumor confined to trachea, > 2 cm T3: Spread outside the trachea but not to adjacent organs or structures T4: Spread to adjacent organs or structures Regional lymph nodes N1: Positive regional nodal disease Distant metastasis M1: Distant metastasis The incidence of multiple primary carcinomas in tracheal carcinoma is 28%. Carcinomas of the larynx, thyroid gland, esophagus, and lung are most commonly responsible for secondary invasion of the trachea. Neoplasms may also involve the trachea by metastasizing from distant sites. |
Benign tracheal tumors | Eccentric, broad-based or pedunculated, well-defined, polypoid mass confined within the tracheal lumen without evidence of adjacent invasion. The lipomas manifest as fat density; the other benign tumors are enhancing soft tissue–density masses. | Benign tumors of the trachea are quite rare (~10% of primary tumors): juvenile laryngotracheal papillomatosis, solitary papilloma, leiomyoma, lipoma, pleomorphic adenoma, granular cell tumor, hemangioma, neurogenic tumor, glomus tumor, fibroma, chondroma, and amyloidoma. These neoplasms may present with symptoms of airway obstruction. |
Paratracheal air cyst | Air cyst, unilocular or multilocular, with rounded margins, 1 to 15 mm in size, in the right posterior paratracheal region at the thoracic inlet, with one or more narrow communicating necks with the trachea. | Relatively common tracheal diverticula (3.7% of the population). Should not be confused with pneumomediastinum in patients who have sustained a traumatic injury, or an apical lung herniation on the right. |
Acquired tracheal stenosis | Acute postintubation stenosis results from mucosal edema or granulation tissue, seen as focal narrowing of tracheal lumen by eccentric or concentric soft tissue thickening internal to normal-appearing tracheal cartilage. Outer tracheal wall has normal configuration, and trachea has normal oval shape. In patients with chronic stricture , the tracheal lumen is narrowed primarily because of collapse and inward displacement of calcified tracheal cartilage. Mucosa and submucosa may be normal or thickened by fibrosis. CT findings of tracheobronchopathia osteochondroplastica include thickened tracheal cartilage with small 3- to 8-mm calcific nodules along its inner aspect, protruding into the tracheal lumen with irregular narrowing. Diffuse amyloidosis leads to concentric, smooth, or nodular thickening of the submucosal tracheal wall. Cartilage is normal, but concentric submucosal calcification or ossification may occur. Respiratory relapsing polychondritis is characterized by wall thickening limited to the anterior and lateral tracheal walls, sparing of the posterior membrane, collapse of the cartilage, and narrowing of the lumen. If cartilage calcification is present, the cartilage may appear thicker than normal. The inner and outer borders of the thickened tracheal wall are smooth. | Symptomatic tracheal stenoses are usually caused by prolonged intubation or tracheostomy. Other rare causes of acquired cervical tracheal stenosis are tracheobronchopathia osteochondroplastica, amyloidosis, and relapsing polychondritis. Wall thickening and narrowing of the tracheal lumen may also be seen in Wegener granulomatosis, miscellaneous inflammatory lesions (sarcoidosis, ulcerative colitis) and various infectious processes (healing stage of tuberculous tracheitis). |












Disease | CT Findings | Comments |
Congenital/developmental lesions | ||
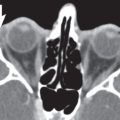
Thyroglossal duct cyst | The infrahyoid thyroglossal duct cyst is typically seen anterior to the larynx as a nonenhancing, smooth, well-circumscribed hypoattenuated mass within or beneath the strap muscles. Occasionally, a thyroglossal duct cyst can protrude into the preepiglottic space through the incisura of the thyroid cartilage. The paraglottic space is spared (as opposed to laryngoceles). | Thyroglossal duct cysts arise from the thyroglossal duct remnant. Other congenital nonneoplastic lesions of the larynx are hamartoma, ectopic thyroid, and thymus. |
Venous vascular malformation (cavernous hemangioma) | Venous vascular malformations appear as well-circumscribed soft tissue masses that display uniform intense contrast enhancement. Phleboliths within the lesion are pathognomonic for venous vascular malformations. | Venous vascular malformations of the larynx typically occur in the subglottis arising from the posterior wall (infantile type) or originate from the true vocal cords or, less common, from the supraglottis (adult type). Hemangiomas in adults may be isolated lesions or associated with cervicofacial angiodysplasia. |
Degenerative/acquired lesions | ||
Laryngocele | Laryngoceles are usually unilateral (75%). An internal (simple) laryngocele is an air–filled, well-defined, thin-walled cystic mass, identified deep to the false cord in the paraglottic space, which can be followed down to the level of the laryngeal ventricle. Less common external laryngoceles protrude through the thyrohyoid membrane into the anterior cervical space with dilation of only the external portion of the lesion. Mixed laryngoceles pass through the thyrohyoid membrane, with dilation of both the interior and exterior segments. A fluid-filled laryngocele is called a laryngeal mucocele: CT density can vary depending on the presence of proteinaceous secretions and other debris. An infected, pus-filled laryngocele with wall thickening and a peripheral rim enhancement is termed a pyolaryngocele. Laryngeal neoplasms may present indirectly with a secondary laryngocele. | Laryngoceles are commonly an acquired expansion of the saccule of the anterior laryngeal ventricle into the paraglottic space of the supraglottic larynx, with gradual enlargement over time, and with or without extension laterally through the thyrohyoid membrane into the anterior cervical space. Presumably they arise from increased intralaryngeal pressure, as in chronic coughers, wind instrumentalists, and glass blowers. A congenital predisposition is considered a likely possibility. Obstruction of the sacculus by tumor, postinflammatory stenosis, trauma, or amyloid is a less common cause of laryngocele (15%). Laryngoceles present commonly in adulthood; more common in men. Many are asymptomatic. When symptomatic, they may be associated with airway obstruction, hoarseness, and dysphagia and present endoscopically as a submucosal supraglottic mass or cause a compressible swelling of the neck. |
Retention cyst | Fluid-filled cystic mass measuring up to several centimeters in diameter with submucosal deformity. Often protrudes into the airway. | Mucosal cysts can arise from obstruction of mucous gland ducts. With the exception of the free margin of the vocal cord, they can occur anywhere in the larynx. A vallecular cyst (oropharyngeal lesion, more common in children) bulges between the base of the tongue and the free anterior margin of the epiglottis, unilateral or bilateral. Congenital laryngeal cysts originate in the aryepiglottic fold and may bulge into the laryngeal vestibule, preepiglottic space, or lateral neck. |
Fibrous and fibroangiomatous polyp | Nodular isodense lesion on the free margin of the true vocal cord, commonly located at the junction of the anterior and middle thirds. Frequently bilateral and occasionally with multiple lesions. | Nonneoplastic stromal reaction in patients with a history of vocal abuse (e.g., singers and professional speakers). |
Gout | May manifest as acute gouty cricoarytenoiditis with vocal fold immobility and asymmetrical soft tissue swelling around the arytenoid cartilages, with airway obstruction, or as chronic tophaceous involvement of the thyroid lamina. | Gouty involvement of the larynx must be considered in any patient with a history of gout who presents with hoarseness, odynophagia, dysphagia, stridor, or neck lump. |
Amyloid | Focal or diffuse laryngeal thickening with nodules, plaques, and calcifications, commonly in the epiglottis, ventricles, and true and false cords. Laryngeal amyloidoma sometimes mimics a laryngocele filled by high-density fluid. | Submucosal amyloid deposition from larynx to distal airways; incidental finding or associated with systemic disease. Usually occurs in the fifth decade of life. Common symptom is hoarseness. |
Inflammatory/infectious conditions | ||
Laryngotracheobronchitis (croup) | Edematous mucosal swelling, most significant in the subglottic area, with steeple-shaped airway narrowing. | Parainfluenza or respiratory syncytial virus infection in young children (3 m–3 y) and, rarely, in adults. The onset is gradual, with several days of upper and lower respiratory tract symptoms followed by the development of a classic barking cough and stridor. |
Acute epiglottitis | Symmetric edematous swelling of the larynx, including the epiglottis, arytenoids, and aryepiglottic folds, with airway obstruction and ballooning of the pharynx. | Caused by Haemophilus influenzae, Pneumococcus, or Streptococcus, usually in children between 1 and 5 y of age. Can cause acute severe respiratory compromise. The term supraglottitis refers to a disease in adults with inflammation of the supraglottis, valleculae, base of the tongue, uvula, soft palate, platysma muscle, and prevertebral soft tissues. Angioneurotic edema has identical presentation on CT. Associated thickening of the retropharynx may be present with angioneurotic edema but not with epiglottitis. |
Tuberculosis (TB) | Laryngeal TB manifests as diffuse bilateral laryngeal thickening with or without a focal mass. Inadequate treatment of TB can lead to laryngeal stenosis or cricoarytenoid fixation. | Results from pooling of infected secretions in the posterior larynx or from hematogenous dissemination to the anterior larynx. A variety of other granulomatous diseases, such as syphilis, fungal infections, sarcoidosis, plasma cell granuloma, Wegener granulomatosis, and Langer-hans cell histiocytosis, may affect the larynx with diffuse or focal laryngeal thickening and ulceration; cannot be differentiated radiologically from neoplasia. |
Rheumatoid arthritis | Cricoarytenoid subluxation, cricoarytenoid and subglottic swelling, sometimes with polypoid rheumatoid nodules. | Laryngeal abnormalities are seen in up to 50% of patients with advanced rheumatoid disease. |
Relapsing polychondritis | Laryngotracheal involvement can be localized or diffuse. Initially, a soft tissue swelling around the laryngeal and tracheal cartilages is present with airway narrowing. Later, the weakened cartilages collapse or are destroyed. Calcification may be seen in the abnormal soft tissues. | Rare connective tissue disease that causes inflammation that may affect cartilage throughout the body. The clinical features include auricular chondritis, arthritis, laryngotracheal symptoms, nasal chondritis, ocular inflammation, and audiovestibular and cardiovascular symptoms. The peak onset is in the fourth and fifth decades; average age is 47 y, with a female predominance of 3:1. |
Benign neoplasms | ||
Papilloma | Solitary or multiple small nodular lesions, frequently located in the anterior half of the larynx at the level of the vocal cords. Subglottic extension with spread to the trachea and bronchi may be associated. | Squamous cell papilloma caused by human papilloma-virus infection is the most common benign laryngeal tumor. It typically occurs in children younger than 10 y with juvenile papillomatosis; solitary in adults. |
Chondroma | Chondromas of the larynx primarily affect the cricoid and thyroid cartilages. The relatively circumscribed, lobulated, hypoattenuating tumor expands the involved site and has stippled or coarse intratumoral calcifications, characteristic of a chondrogenic tumor. However, there are no reliable CT criteria that enable differentiation between chondrosarcoma and benign chondroma. | True chondromas of the larynx are extremely unusual and are greatly outnumbered by laryngeal chondrosarcoma. They may be an incidental finding or cause minor symptoms, such as hoarseness. A clinically significant cartilaginous neoplasm > 2 cm in dimension more likely represents laryngeal chondrosarcoma. Other benign tumors of the cartilaginous skeleton occur but are even more unusual, including aneurysmal bone cyst, giant cell tumor, aggressive osteoblastoma, and brown tumor. |
Lipoma | Laryngeal lipoma commonly arises in the supraglottic region and typically manifests as a homogeneous, nonenhancing lesion with attenuation values from –65 to –125 HU. Internal architecture is minimal. | Laryngeal lipomas comprise < 0.5% of benign neoplasms at these sites, occur in all ages, and affect both genders equally. The symptoms are nonspecific but often include airway obstruction. At endoscopy, the tumor may manifest as a solitary, sessile, or pedunculated polypoid submucosal mass. |
Paraganglioma | Occurs in the supraglottic larynx and presents as a submucosal mass in the region of the aryepiglottic fold–false vocal cord. The right side of the larynx is more often involved. On CT, the highly vascularized neoplasm shows homogeneous intense enhancement. | Laryngeal paragangliomas are very rare. These neuroendocrine tumors are three times more common in women and have been described in patients from 5 to 83 y of age (median age 44 y). Patients present with hoarseness, dysphagia, dyspnea, stridor, hemoptysis, and foreign body sensation in the throat. Functioning laryngeal paragangliomas are exceptional. |
Nerve sheath tumors (schwannoma, neurofibroma) | Present as round, well-defined supraglottic masses hypoattenuated on unenhanced CT images and slightly enhanced with intravenous (IV) administration of contrast material. A target appearance with central nonenhancing and peripheral enhancing areas may be seen. | Laryngeal nerve sheath tumors are very unusual. These smooth-surfaced submucosal tumors arise from the superior laryngeal nerve, single or multiple, unilateral or bilateral. They occur as isolated cases or in patients with von Recklinghausen disease and neurofibromatosis 2. Dysphonia and hoarseness may be the presenting symptoms. Other submucosal benign neoplasms of the larynx (e.g., leiomyoma, rhabdomyoma, ganglioneuroma, and granular cell tumor) occur but are even more unusual. |
Malignant neoplasms | ||
Squamous cell carcinoma | The imaging criteria used to define the site and the extent of tumor consist of the detection of a solid mass projecting into the lumen, thickening of soft tissues, abnormal enhancement, and fat tissue effacement. The imaging that correlates to tumoral fixation of the cord includes cricoarytenoid joint involvement, interarytenoid disease, and paraglottic spread. Extralaryngeal tumor and eroded edges or lysis of thyroid cartilage and sclerosis or lysis of cricoid and arytenoid cartilage should raise suspicion for cartilage invasion. | Over 95% of laryngeal tumors are squamous cell carcinomas. They occur mainly in men who abuse tobacco and alcohol. Glottic carcinomas (60%) are diagnosed early due to hoarseness. Supraglottic (30%) and subglottic (5%) carcinomas are symptomatic late in the course of the disease and therefore usually present in the advanced stage. Their submucosal extension cannot be evaluated by endoscopic examination alone. Carcinoma of the larynx: TNM classification Primary tumor |
Supraglottic carcinomas originating from the epiglottis primarily invade the preepiglottic space. Tumors that originate from the petiole often invade the low preepiglottic space and, via anterior commissure, the glottis or subglottis. Tumors originating from the aryepiglottic fold, false cord, and laryngeal ventricle primarily infiltrate the paraglottic space. Tumors arising in the arytenoid and interarytenoid region usually infiltrate submucosally toward the hypopharynx. Supraglottic carcinoma tends to spread into the pyriform sinus or toward the base of the tongue, but it rarely invades the glottis and thyroid cartilage. Deep cervical lymph node metastases occur early and are often bilateral. | Supraglottis T1: Tumor confined to one subsite* T2: Tumor invades mucosa of more than one subsite* or glottis or region outside the supraglottis with normal cord mobility T3: Tumor limited to the larynx with fixation of vocal cord and/or extension into the postcricoid area, medial wall of pyriformis sinus, or preepiglottic space or slight erosion of thyroid cartilage T4a: Tumor invades through thyroid cartilage and/or extends beyond the larynx: trachea, soft tissues of the neck, extrinsic tongue muscles, thyroid gland, esophagus, strap muscles T4b: Tumor invades prevertebral space, mediastinum, or encases carotid artery | |
Glottic carcinoma typically arises from the anterior half of the vocal cord and primarily spreads into the anterior commissure, which should not exceed 1 mm in thickness. From the anterior commissure, glottic tumors may grow into the infra- or supraglottic regions or into the contralateral vocal cord, or they gain access via the thyroid cartilage and cricothyroid membrane to the prelaryngeal soft tissues of the neck. Alternatively, tumor may grow posteriorly to the posterior commis-sure, arytenoids, cricoarytenoid joints, subglottic space, or postcricoid region. Lymphatic metastases from localized glottic carcinoma are uncommon. | * Subsites include epiglottis, aryepiglottic folds, and false cords, including the laryngeal ventricles. Glottis T1: Tumor confined to one (T1a) or both vocal cords (T1b), including anterior or posterior commissures with normal cord mobility T2: Tumor extends to supraglottis and/or subglottis and/or associated with impaired vocal cord mobility T3: Tumor limited to the larynx with vocal cord fixation, and/or invades paraglottic space, and/or inner cortex of thyroid cartilage | |
Subglottic carcinoma is diagnosed by demonstrating any soft tissue inside the cricoid cartilage, as there is no normal tissue between the mucosal surface and cricoid cartilage. The tumor may spread to the vocal cord and supraglottis, thyroid gland, hypopharynx, cervical esophagus, and tracheal wall. Subglottic carcinoma is very often accompanied by lymph node metastases, both paratracheal and pretracheal. | T4a: Tumor invades through thyroid cartilage and/or extends beyond the larynx: trachea, soft tissues of the neck, extrinsic tongue muscles, thyroid gland, esophagus, strap muscles T4b: Tumor invades prevertebral space, mediastinum, or encases carotid artery Subglottis T1: Tumor confined to subglottis T2: Tumor invades vocal cord (s) with normal or impaired mobility T3: Tumor confined to larynx with vocal cord fixation T4a: Tumor invades through cricoid or thyroid cartilage and/or extends beyond the larynx: trachea, soft tissues of the neck, extrinsic tongue muscles, thyroid gland, esophagus, strap muscles T4b: Tumor invades prevertebral space, mediastinum, or encases carotid artery Regional lymph nodes N1: Single ipsilateral node < 3 cm N2a: Single ipsilateral node 3 to 6 cm N2b: Multiple ipsilateral nodes < 6 cm N2c: Bilateral or contralateral nodes < 6 cm N3: Node (s) > 6 cm Distant metastasis M1: Distant metastasis | |
Laryngeal chondrosarcoma | Laryngeal chondrosarcomas arise predominantly in the ossified hyaline cartilages. The posterior and posterolateral aspect of the cricoid is most frequently involved, followed by the thyroid cartilage (inferolateral); the arytenoid cartilage, epiglottis, and hyoid bone are rare locations. CT shows an expansile solid hypodense (to muscle), moderately enhancing mass with intrinsic arc and ringlike, stippled, coarse or “popcorn” calcifications and cortical destruction. The tumor may remain endolaryngeal and lead to significant airway narrowing or more commonly extend into the immediate exolaryngeal soft tissues. | Sarcomas of the larynx are extremely rare neoplasms that account for ~1% of all tumors of this organ. Chondrosarcoma is the most common sarcoma of the larynx and predominantly affects men in their sixth or seventh decade of life. Patients present with progressive hoarseness, dyspnea, stridor, or dysphagia. An indolent external neck mass may be palpable. At endoscopy, the tumor may manifest as a solitary, lobulated submucosal mass with intact mucosal surfaces. Other malignant tumors of the cartilaginous skeleton occur, including osteosarcoma and multiple myeloma, but they are even more unusual. |
Malignant minor salivary gland tumor | None of these unusual types of submucosal carcinomas have any imaging characteristics to distinguish them from squamous cell carcinoma. | Malignant minor salivary gland tumors may arise from the minor salivary glands in the supraglottic and subglottic regions. Adenoid cystic carcinoma most commonly arises in the subglottis, invades the entire larynx submucosally, and infiltrates the thyroid gland and the esophagus. Common symptoms include pain and paralysis of the recurrent laryngeal nerve. No gender predilection. Mucoepidermoid carcinoma: Men are a ffected six times more often than women; the epiglottis is the most commonly affected site. Adenocarcinoma: Most commonly found in the fifth to seventh decade with a male predominance. Presents with extensive submucosal tumor spread and invasion of the laryngeal skeleton. |
Non–Hodgkin lymphoma | Laryngeal non–Hodgkin lymphoma is typically a submucosal homogeneously enhancing soft tissue mass centered in the supraglottis and may spread to involve the glottis and subglottis. Deep tumor invasion into cartilage or strap muscles may occur, as well as cervical lymphadenopathy. Involvement of the hypopharynx and superior extension to the oropharynx or even nasopharynx should raise the possibility of non–Hodgkin lymphoma. | Non–Hodgkin lymphoma of the larynx is rare. Patients with laryngeal lymphoma (M:F = 1:3, mean age 58 y) commonly present with progressive hoarseness, cough, dysphagia, or, less frequently, systemic symptoms. |
Metastases to the larynx | Melanoma and renal adenocarcinoma usually metastasize to the soft tissues, mainly the vestibular and aryepiglottic folds. Lung and breast carcinomas may metastasize to the marrow spaces of the ossified thyroid, cricoid, and arytenoid cartilages with destruction of the laryngeal skeleton. | Metastases to the larynx are rare, most often found in men. The primary sources of metastatic tumor are skin, kidney, breast, lung, prostate, colon, stomach, and ovary. Common sites of involvement are the supraglottic and subglottic regions. |
Trauma | ||
Dislocation of joints | Dislocation of the cricoarytenoid joint is straightforward to diagnose due to the abnormal position of the arytenoid relative to the cricoid cartilage (the arytenoid cartilage is tipped forward and rotated medially), edema of the aryepiglottic fold, and bowed hypomobile vocal cord. Cricothyroid dislocation appears as a rotation of the cricoid ring relative to the thyroid with widening of the space between the lower thyroid and the cricoid. It is most commonly associated with thyroid or cricoid fracture. | The mechanism of external laryngeal injury can be divided into blunt trauma, in which the larynx and upper trachea are crushed against the spine, and penetration trauma. Blunt trauma tends to be associated with motor vehicle accidents, sports injuries, falls, and strangulation, whereas penetrating trauma is related to assaults. Internal laryngeal injury is related to intubation, instrumentation, ingestion from foreign bodies and caustic substances, and radiation. |
Cartilage fracture | Fractures of the thyroid cartilage may be vertical or horizontal, or the entire thyroid cartilage may be shattered with dislocation of fragments of cartilage. Fractures of the cricoid cartilage tend to occur bilaterally and lead to the collapse of the cricoid ring. Fractures are invariably associated with soft tissue abnormalities (subcutaneous emphysema, mucosal tears, edema, and hematoma with loss of internal laryngeal landmarks). | A hyoid fracture is often associated with avulsion of the posteriorly displaced epiglottis. Laryngotracheal separation shows malalignment between the larynx and trachea |
Submucosal hematoma | Distention and increased soft tissue densities in the preepiglottic and paraglottic spaces, swelling of the aryepiglottic folds, true and false vocal cords, and increased soft tissue densities within and around the cricoid cartilage. | Endolaryngeal soft tissue hemorrhages are usually associated with trauma, with or without cartilage damage. Spontaneous hemorrhage occurs with bleeding disorders and anticoagulation. |
Vocal cord paralysis | CT imaging features are explained by atrophy of the thyroarytenoid muscle and include an enlarged ventricle, ipsilateral enlargement of the pyriform sinus, thickening and anteromedial displacement of the aryepiglottic fold, and paramedian position, decreased size, and fatty infiltration of the true vocal cord. Left is more common than right. | Immobilization of true vocal cord by denervation. Many cases are idiopathic, toxic, or inflammatory/infectious etiologies. Lesions compressing or injuring vagus or recurrent laryngeal nerves are surgery, trauma, and masses, both cancerous and noncancerous. In a patient with vocal cord paralysis of unknown origin, CT should be extended to the skull base and the mediastinum to include the entire pathway of the vagus and recurrent laryngeal nerve. |
Subglottic stenosis | Eccentric or circumferential subglottic or tracheal luminal narrowing with wall thickening due to submucosal edema, granulation tissue, or collapsed cartilage. | Congenital or acquired (e.g., complication of prolonged intubation or tracheostomy, or sequelae of cricoid cartilage fracture). |














Disease | CT Findings | Comments |
Pseudomass | ||
Pyramidal lobe of the thyroid gland | An accessory lobe usually arises from the isthmus and extends superiorly, superficial to the thyroid cartilage, along the course of the distal thyroglossal duct. It may be attached to the hyoid bone. Uncommonly, the pyramidal lobe may arise from the medial right or left thyroid lobe. | Normal variant. May be present in 10% to 40% of the population. A pyramidal lobe is most commonly recognized in patients with Graves disease. |
Prominent thyroid isthmus | Infrequently, the thyroid isthmus may reside anterior to the cricoid cartilage. | The thyroid isthmus (1.25–2 cm in width and height, < 0.6 cm thick) is a midline structure, anterior to the trachea (usually overlying the first through third tracheal rings). |
Congenital/developmental lesions | ||
Ectopic thyroid | Ectopic thyroid tissue is dense (80–100 HU) because of its iodine content and typically enhances markedly on postcontrast CT. | Any disruption of thyroid descent may lead to either lingual thyroid, seen with complete failure of descent, or ectopic thyroid, with thyroid tissue anywhere along the course of the thyroglossal duct. Overdescent of the thyroid may result in ectopic thyroid in the mediastinum, on rare occasions in the trachea or heart. Ectopic thyroid is subject to the same diseases as the anatomically correctly positioned thyroid. Development of a mass lesion is often the reason why these ectopic thyroids become symptomatic. |
Thyroglossal duct cyst | Nonenhancing, smooth, thin-walled, low-density (cystic) round or ovoid midline neck mass (usually 2–4 cm), occasionally septated, located either at or below the level of the hyoid bone (80%), or suprahyoidal at the base of the tongue or within the posterior floor of the mouth (20%). The more inferior the cyst, the more likely it is to be offthe midline, deep to or embedded in the infrahyoid strap muscles (“claw” sign). The wall may thicken and enhance and the cyst content develop higher attenuation, if infected. Any associated nodularity or chunky calcification within the cyst suggests associated thyroid carcinoma. Occasionally associated with thyroid a genesis, ectopia, and pyramidal lobe. | Failure of the hollow thyroglossal duct to involute may result in a persistent fistulous tract or cyst along the path of migration between the foramen cecum and thyroid bed in the infrahyoid neck. Thyroglossal duct cysts are the most common embryologic remnant in the neck, usually detected before the age of 20 y, frequently following infection. There is no gender predilection. |
Thyroid hemiagenesis | Absence of a thyroid lobe and enlargement of the contralateral lobe. | Thyroid agenesis is uncommon. Hemi-agenesis is also rare; however, when it does occur it often involves the left lobe. Occasionally the isthmus may be absent. |
Inflammatory/infectious conditions | ||
Hashimoto thyroiditis | Diffuse moderately enlarged, lobular, generally hypodense thyroid gland with mild heterogeneity and less well-defined margins. No calcifications or areas of necrosis or adenopathy are evident. In 12%, there may be atrophy and fibrosis of the gland. | Chronic, autoimmune-mediated lymphocytic inflammation of thyroid gland, leading to gland destruction and hypothyroidism; a ssociated with an elevated risk of thyroid gland lymphoma and papillary carcinoma. It occurs most often in women over the age of 40 y. Familial predisposition. |
De Quervain thyroiditis | Usually mild unilateral or bilateral and symmetric thyroid enlargement with diffusely decreased attenuation (isodense/hypodense to muscle) and only moderate contrast enhancement. There may be atrophy of the thyroid gland over time. | Uncommon, subacute, presumably viral thyroiditis presenting with painful gland enlargement, fever, and fatigue after an upper respiratory tract infection. Most commonly affects middle-aged women. Hyperthyroidism is present in half of all patients, sometimes followed by transient hypothyroidism. It is a self-limited disease; complete recovery in weeks to months is characteristic. |
Riedel thyroiditis | Unilateral or bilateral, often asymmetric and irregular enlargement of a diffusely hypodense thyroid gland. Decreased contrast enhancement increases the contrast between the residual normal thyroid tissue and the fibrotic parenchyma. Compression of the trachea, esophagus, and vessels and/or obliteration of the adjacent soft tissue planes may simulate an infiltrative mass. | Extremely rare form of chronic thyroiditis (immune-mediated?), characterized by replacement of the normal thyroid parenchyma by a dense fibrosis that extends beyond the thyroid capsule and invades adjacent structures of the neck. Multifocal fibro-sclerosis (retroperitoneal and mediastinal fibrosis, sclerosing cholangitis, and orbital pseudotumor) may be associated. Occurs preferentially in women around 50 y of age. Patients present with an enlarging mass causing compression of the trachea, hoarseness, difficulty in swallowing, and hypothyroidism. |
Suppurative thyroiditis | Neck and glandular swelling secondary to edema with hazy lobe margins and a low-density parenchymal mass, usually unilateral, with left-sided predominance. In the presence of an abscess, a single or multiloculated area of fluid density with or without air and air–fluid levels may be identified in the thyroid or in the perithyroid soft tissues of the neck with peripheral rim enhancement. | Acute suppurative thyroiditis is uncommon, mainly caused by Streptococcus haemolyticus, Staphylococcus, and Pneumococcus. Can occur in immunosuppressed persons, but also in otherwise healthy patients after trauma or irradiation. Preexisting thyroid disease, particularly nodular goiter, is present in 50% of adult patients. A special form is recurrent infection caused by a pyriform fossa sinus tract, as found in third or fourth branchial cleft anomalies, or by a thyroglossal duct fistula. Patients present with acute anterior neck pain, fever, and dysphagia. These patients usually have euthyroidism. |
Degenerative lesions | ||
Thyroid cyst | Unilateral, large cystic mass within normal thyroid tissue. Cysts are usually hypodense but become isodense when the protein content, including thyroglobulin, is elevated. The wall is thin and smooth. Bleeding may occasionally occur into a cyst, resulting in a sudden increase of the cyst. | Thyroid cysts account for 15% to 25% of all thyroid nodules. True thyroid cysts lined with epithelium are rare. Most cysts are the result of degeneration of thyroid adenomas, with the accumulation of serous fluid, colloid substance, or blood. A cervical thymic cyst in contiguity with the lower pole of the thyroid may mimic a thyroid cyst. |
Amyloid deposition | Diffuse glandular enlargement. The enlarged cervical lymph nodes may be homogeneously enhanced. Involvement of the airways shows subglottic and tracheal narrowing. | Amyloidosis involving the thyroid gland is very rare and may be seen both in patients with Hashimoto thyroiditis and in patients with systemic amyloidosis. Amyloid deposition in systemic amyloidosis may also be seen in the cervical lymph nodes, larynx, and trachea. |
Metabolic disorders | ||
Multinodular goiter | Well-marginated, diffuse, asymmetric enlargement of the thyroid gland with heterogeneous appearance. Degenerative and colloidal cysts appear as multiple hypodense areas; solid adenomatous nodules and fibrosis contribute to variably sized, intermediate-attenuation masses; and hemorrhage has a high density. Focal amorphous, ringlike, and curvilinear calcifications are present in 90%. Substernal and mediastinal extension occurs in 37% of patients. Secondary manifestations of goiter include compression and displacement of the trachea, esophagus, and adjacent vessels. No associated lymphadenopathy. | Goiter refers to any enlargement of the thyroid gland, related to genetic and environmental factors. Endemic goiters are prevalent in iodine- deficient areas. Most simple diffuse goiters progress to multinodular goiter. There is a 2 to 4:1 female predominance, and the incidence grows with age. Patients usually present with local compression symptoms and cosmetic disfigurement. Multinodular goiters may or may not be associated with functional thyroid abnormalities. In toxic nodular goiter (Plummer disease), hyperthyroidism is caused by the autonomous function of one or more adenomas. Anaplastic thyroid carcinoma may arise from multinodular goiter in 5% (risk factors: radiation exposure, family history of thyroid carcinoma, and rapid growth). |
Benign neoplasms | ||
Thyroid adenoma | Inhomogeneous hypodense mass, 1 to 4 cm in diameter, within the focal enlarged thyroid gland. Margins are well circumscribed. Large adenomas enhance more heterogeneously because of hemorrhage, cyst formation, fibrosis, and multiple amorphous calcifications within it. Absence of invasive features and lymphadenopathy suggests thyroid adenoma (biopsy is always required to rule out malignancy). Thyroid nodules with calcifications in males, thyroid nodules with blurred calcifications, and calcifications in the margin of the lesion may suggest malignant disease. | True benign neoplasm of differing histopathologic types, usually solitary and nonfunctioning, often incidentally detected in young and middle-aged adults (M:F = 1:4). Most thyroid cysts represent spontaneous degeneration of adenomas. Sudden enlargement of an adenoma is usually related to spontaneous hemorrhage within the lesion. A toxic adenoma is an autonomous functioning adenoma that usually exceeds 3 cm in diameter before it produces clinically apparent hyperthyroidism. The colloid or adenomatous nodule is composed of focal hyperplastic epithelium and is not a true neoplasm. Rarely, a parathyroid adenoma has an ectopic intrathyroid location. Thyroid nodules are common and comprise adenomas, cysts, focal thyroiditis, multinodular goiter, and malignant tumors. The incidence of malignancy in a single nodule is 10% to 15%, in a multinodular goiter 4% to 7.5%. Most thyroid cancers present as cold nodules on radionuclide scans. |
Malignant neoplasms | ||
Papillary carcinoma | The primary tumor appearance is highly variable. The tumor is isodense with muscle interspersed with low-attenuation areas representing cystic or necrotic areas. Psammomatous or amorphous calcifications are present in up to 35% of patients. The size of the tumor ranges from small to large. The neoplasm may be multifocal. Less commonly, the tumor may be diffuse and invasive with extrathyroidal extension. Aggressive forms may invade the larynx and trachea or esophagus. Metastases to lymph nodes may be solid and hypervascular, solid and cystic, or cystic and display calcifications. In some cystic metastases, the wall may be smooth and thin, simulating a cystic lesion. | Papillary carcinoma is the most common thyroid cancer (60%–80%, peak incidence in the third and fourth decades, with a female predominance [3:1]). As many as 20% are multifocal. In up to 50% lymph node metastases may precede the clinical recognition of a tumor in the thyroid gland and may occur in the paratracheal and supraclavicular areas (l evels IV–VI), midjugular and upper jugular nodes (levels III and II), as well as in retropharyngeal and superior mediastinal nodes. Distant metastases (lung, bone) occur in 5% to 7%. |
Follicular carcinoma | The tumor appearance is a reflection of the pathologic changes ranging from a well-delimited nodule to an ill-defined invasive tumor with necrosis and calcification. They rarely become cystic. A small percentage invades the larynx, trachea, and esophagus. Nodal spread is rare (10%); distal spread is more common (20%). | Follicular carcinoma constitutes ~20% to 25% of thyroid carcinomas, has a female predominance, and occurs a decade later than papillary carcinoma, with a peak incidence in the fifth decade. The majority of follicular carcinomas are slow growing, solitary, and encapsulated, resembling an adenoma. The widely invasive form extends outside the gland and penetrates the surrounding tissue. |
Anaplastic carcinoma | Usually large, irregular, heterogeneously dense-hyperdense thyroid mass with central low-attenuation areas, reflecting hemorrhage and necrosis (75%), and dense amorphous, globular calcifications (58%). Extrathyroidal extension with invasion of neighboring soft tissue structures, aerodigestive system, carotid artery, and jugular vein is common (26%–53%). Mediastinal extension is encountered in 25%. Lymph node metastases (40%), often necrotic, and distant hematogenous metastases (50%) are common. | Anaplastic (undifferentiated) carcinoma accounts for < 5% of thyroid malignancies. It is the most aggressive neoplasm in the thyroid and has a female predominance, with a peak incidence in the sixth or seventh decade. In almost 50% of cases, the anaplastic carcinoma develops within a goiter or may arise from differentiated thyroid cancer. |
Medullary thyroid carcinoma | Solid nonenhancing, low-density, well-circumscribed mass in the thyroid gland, frequently multifocal and bilateral. Fine, punctate, or coarse calcifications may be found in the tumor and in nodal metastases. | Rare neuroendocrine malignancy arising from thyroid parafollicular cells (4%–5% of all thyroid gland malignancies). Although medullary cancer is associated with multiple endocrine neoplasia type 2 (MEN 2) syndromes and hereditary, familial forms, 80% occur sporadically. Patients who have sporadic medullary carcinoma typically present with a painless palpable nodule in the fifth or sixth decade of life, with bilateral tumors in 20%, but up to 75% have lymphadenopathy at presentation. There is a slight female preponderance (1.5:1). MEN 2 syndromes occur in children and younger adults (mean age 35 y), with multifocal and bilateral manifestation in 80%. Distant metastases may settle in the lungs, liver, and bone. |
Lymphoma | The CT aspect is variable and includes an isolated nodule (80%), bilateral nodules (20%), or bulky mass involving the entire gland. The tumor is often homogeneous, solid, and hypodense and shows only minor enhancement. Necrosis and calcifications are uncommon. A diffusely infiltrated gland results in a hypodense thyromegaly with extraglandular extension. When present, enlarged cervical lymph nodes are usually multiple, bilateral, nonenhancing, hypodense, and solid. | Thyroid non–Hodgkin lymphoma (2%–5% of all thyroid malignancies; M:F = 1:4; peak incidence sixth decade) is either primary (defined as an extranodal, extralymphatic lymphoma that arises from the thyroid gland) or secondary as part of systemic lymphoma. Most thyroid lymphomas are non-Hodgkin B-cell lymphomas. Less common are mucosa-associated lymphoid tissue (MALT) lymphomas, rarely Hodgkin lymphoma, Burkitt cell lymphoma, and T-cell lymphoma. Patients with Hashimoto thyroiditis are predisposed to thyroid gland lymphoma. Patients usually present with a rapidly enlarging thyroid mass and obstructive symptoms related to compression of the aerodigestive tract. |
Metastases to the thyroid gland | Single or multiple thyroid masses of variable size demonstrating low density. In advanced forms, the entire thyroid gland may be invaded, indistinguishable from a primary thyroid tumor. | Metastatic disease to the thyroid gland usually is clinically occult, although 2% to 4% of patients dying of malignant disease (skin [metastatic melanoma], breast, lung, kidney, or colon) may present metastases at autopsy. A separate category includes malignant tumors that invade the thyroid from adjacent structures, such as carcinomas of the larynx, pharynx, trachea, and esophagus. |








Disease | CT Findings | Comments |
Pseudomass | ||
Carotid bulb asymmetry | Unilateral or asymmetric prominent carotid artery bifurcation without thrombus in older patients with atherosclerosis. | The carotid bulb is the most proximal aspect of the cervical internal carotid artery. The bulb may form a significant focal dilation where the internal carotid artery originates from the common carotid artery at a slight angle. |
Carotid artery ectasia | Round, enhancing structure in the widened retropharyngeal space. Sectional imaging, reconstructions, and computed tomography angiography (CTA) easily define the nature of the retropharyngeal structure as a tubular, tortuous, sometimes dilated carotid artery. | One or both distal common carotid arteries may have a retropharyngeal course. Increasing incidence of medial loop with increasing age. This normal variant may present clinically as a pulsatile retropharyngeal mass. |
Cervical aortic arch | Abnormal, enhancing, tubular structure, continuous with the ascending and descending aorta. Its apex lies well above the medial ends of the clavicles, on the right or on the left side of the vertebral column. In both instances, branching of the arch vessels is variable and may be aberrant. Frequently, the descending aorta is contralateral to the aortic arch. | In this very uncommon congenital anomaly, the aortic arch lies above the manubrium and may present as a pulsatile neck mass; > 80% are right-sided. Patients often have a benign or a symptomatic course, but it may occur with other cardiovascular congenital abnormalities. Only women with cervical aortic arch may develop aneurysms. |
Jugular vein asymmetry | The size of the internal jugular veins can be variable and asymmetric due to their reciprocal size relationship to the external jugular veins and positional and anatomical factors. The right internal jugular vein is usually larger than the left. | Incidental finding on CT without any pathologic significance. Sometimes a large vein can be felt in the neck as a soft mass. Phlebectasia of the internal jugular vein is a rare benign focal fusiform dilation of the inferior aspect of the internal jugular vein with enlargement during Valsalva maneuver and decrease at rest, usually seen in children and young adults. Phlebectasia should be differentiated from true venous aneurysms. |
Congenital/developmental lesions | ||
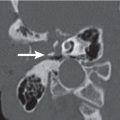
Atypical second branchial cleft cyst | Rarely, a second branchial cleft cyst can extend into the infrahyoid neck but still maintain close proximity to the carotid artery and internal jugular vein. CT reveals a well-circumscribed, ovoid or rounded, homogeneously hypoattenuated, unilocular mass, surrounded by a uniform thin and regular wall. The mural thickness may increase after infection. A focal projection of cyst extending between the carotid bifurcation, termed a “notch” sign, is considered pathognomonic for a second branchial cleft cyst. | Second branchial cleft cysts can occur anywhere along the line from the tonsillar fossa to the supraclavicular region. They may manifest as an often chronic, recurrent, painless, compressible neck mass in a child or young adult, increasing in size with upper respiratory tract infections. |
Inflammatory/infectious conditions | ||
Reactive lymphadenopathy | In reactive adenopathy, the infrahyoid deep cervical lymph nodes (levels III and IV) may by definition enlarge to a maximum diameter of 1 cm but maintain their normal oval shape, isodensity to muscle, and their homogeneous internal architecture with variable, usually mild enhancement. Enhancing linear markings within the node may be seen. Postinflammatory fatty infiltration appears as low-density nodal hilus, mimicking necrosis, in a node with pronounced lima bean shape. | The internal jugular nodal chain is closely a ssociated but not in the carotid space. Reactive hyperplasia is a nonspecific lymph node reaction, current or prior to any inflammation in its draining area. Can occur at any age but most common in the pediatric age group. |
Viral lymphadenitis | Viral adenitis may present as bilateral diffuse lymph node enlargement without necrosis, similar in appearance to lymphoma and sarcoidosis. | Nonspecific imaging finding that may be observed in a variety of viral infections, such as adenovirus, rhinovirus, enterovirus, measles, mumps, rubella, varicella zoster, herpes simplex virus, Epstein–Barr virus, and cytomegalovirus. However, in the presence of parotid lymphoepithelial cysts and hyperplastic adenoids, human immunodeficiency virus (HIV) infection should be strongly suggested. |
Suppurative lymphadenitis | In suppurative adenopathy, the involved nodes are enlarged, ovoid to round, with poorly defined margins and surrounding inflammatory changes. Contrast-enhanced CT images show thick enhancing nodal walls with central hypodensity, similar to meta-static adenopathy with necrotic center. Extracapsular spread of infection from the suppurative lymph nodes may result in abscess formation. | Common in pediatric age group with acute onset of tender neck mass and fever. Organisms have predilection for specific ages (infants: Staphylococcus aureus, group B, Streptococcus, Kawasaki disease; children 1–4 y: S. aureus, group A, Streptococcus, atypical mycobacteria; children 5–15 y: anaerobic bacteria, toxoplasmosis, cat scratch disease, tularemia; immunocompromised adults: histoplasmosis, coccidioidomycosis, Cryptococcus, Pneumocystis carinii, and toxoplasmosis). |
Tuberculous lymphadenitis | Tuberculous nodes are multiple in presentation, most often bilateral, and may have a variable appearance, ranging from mild reactive hyperplasia to frank caseation and necrosis. Some enlarged nodes may enhance, some may be isoattenuated with muscle, and some may be of a lower attenuation than muscle. Although there may be effacement of the fat planes in the immediate region of the involved nodes, there usually is little infiltration of the adjacent neck. The presence of a multichambered, low-density nodal mass with ringlike areas of enhancement both within and around the mass and a large, low-density mass with a thick, sometimes corrugated rim of enhancement about the periphery (“cold abscess”) are highly suggestive of tuberculous lymphadenitis. Fibrocalcific changes occur in the chronic phase or after treatment. | Tuberculous lymphadenitis occurs when mycobacteria involve the nodes of the infrahyoid neck chains. Nontuberculous mycobacterial infection is the most common cause of granulomatous disease in children, caused by Mycobacterium avium intracellulare, M. bovis, M. scrofulaceum, and M. kansasii. Atypical mycobacterial diseases have a propensity to be unilateral or asymmetric. The imaging findings are similar to those of TB. |
Cat scratch disease | Contrast-enhanced CT may demonstrate enlarged lymph nodes with surrounding edema and areas of central hypodensity, due to necrosis. | Cat scratch disease is a self-limiting infectious disease, caused by Bartonella henselae that may follow the scratch or bite of a cat or other animal. Following inoculation, an erythematous papule forms; several weeks later, a painful regional lymphadenopathy develops in up to 30% of cases. |
Kimura disease | Marked contrast enhancement of enlarged round cervical lymph nodes. | Unusual inflammatory disease with cervical lymphadenopathy, involvement of parotid and submandibular regions, peripheral eosinophilia, and elevated serum immunoglobulin E (IgE) that occurs predominantly in Asian men in their second and third decades. |
Kawasaki syndrome | Unilateral or bilateral cervical lymphadenopathy with enlarged oval reactive nodes. | Kawasaki disease is an acute systemic inflammatory condition that occurs in children younger than 10 y with high fever, oral cavity mucositis, rash, nonpurulent conjunctivitis, cardiac manifestations, and cervical lymphadenopathy (may be seen in 50%–70%). |
Histiocytic necrotizing lymph-adenitis (Kikuchi–Fujimoto disease) | Generally unilateral cervical lymphadenopathy with multiple enlarged oval nodes, with or without necrosis. | Kikuchi–Fujimoto disease is a rare inflammatory disease, typically seen in women in their third decade of life with tender cervical lymphadenopathy, fever, flulike symptoms, leukopenia, generalized lymphadenopathy, and hepatosplenomegaly. |
Posttransplantation lymphoproliferative disorder | Presents in the neck either with diffuse bilateral nodal enlargement or a large nodal mass with central low attenuation. | In 1% to 10% of patients following organ transplantation, lymphadenopathy may be seen, but disease in the neck is uncommon. Can be associated with a mass lesion in Waldeyer ring. |
Abscess | Poorly marginated soft tissue mass in the expanded carotid space with single or multiloculated, low-density center, with or without gas collections, and usually thick abscess wall. Contrast-enhanced CT images show a thick, irregular peripheral rim enhancement and enhancement of the inflamed adjacent soft tissues. | Carotid space abscesses commonly arise from extracapsular spread of suppurative lymphadenitis of the internal jugular chain or are due to penetrating trauma, vascular surgery, and intravenous (IV) drug abuse. |
Nodal sarcoidosis | Multiple, most often bilateral, enlarged, homogeneously enhancing nodes, with sharp margins and a “foamy” aspect. Eggshell calcification is less frequently in the neck. Positive mediastinal nodes are common. | Sarcoidosis is a chronic multisystem granulomatous disease. The most common manifestations in the head and neck include parotid gland, ocular, and lacrimal gland involvement, as well as facial nerve and cervical lymph nodes. |
Giant lymph node hyperplasia (Castleman disease) | Large, single or multiple conglomerate, nonnecrotic nodes; often show massive contrast enhancement. Calcifications may be seen occasionally. | Castleman disease is a rare nodal disease with benign lymphoid tissue hyperplasia, most commonly in the mediastinum (70%); 14% occur in the head and neck, mainly in the cervical nodes, parotid and submandibular regions, and Waldeyer ring. Castle-man disease may also occur as a soft tissue mass in extranodal neck sites. It typically affects men younger than 30 y. |
Sinus histiocytosis (Rosai–Dorfman disease) | Very large, round, nonnecrotic cervical nodes bilateral with homogeneous contrast enhancement. | Rosai–Dorfman disease is a rare benign histiocytic proliferation occurring most commonly in the first two decades of life with massive painless cervical lymphadenopathy. Extranodal disease is common in the upper respiratory tract, orbit, and salivary glands. |
Vascular lesions | ||
Jugular vein thrombosis | Jugular vein thrombophlebitis: Enlargement of the internal jugular vein by intraluminal hyperdense acute thrombus, increased density in fat, and loss of soft tissue planes surrounding the thrombus-filled vein from edema/cellulitis. After IV administration of contrast material, the thickened vessel wall may enhance intensely, contrary to the nonenhancing luminal thrombus. Edema fluid may be present in the retropharyngeal space. Jugular vein thrombosis: The vein is dilated, with low-attenuation intraluminal content and contrast-enhancement of the wall without adjacent inflammation. Collateral veins bypassing the thrombosed jugular vein may be seen. | In the acute-subacute thrombophlebitis phase (< 10 d after acute event), the clinical findings are most commonly those of a swollen, hot, tender, ill-defined, nonspecific neck mass with fever. In the chronic jugular vein thrombosis phase (> 10 d after acute event), patients present with a tender, vague, deep, nonspecific neck mass, in comparison to a palpable cord encountered when the external vein is thrombosed. A history of central venous catheter placement, pacemaker insertion, previous neck surgery, local malignancy, infective cervical lymphadenopathy, deep space infection, polycythemia, or drug abuse is often available. The hallmarks of Lemierre syndrome are septic jugular vein thrombosis after a primary oropharyngeal infection and metastatic infection. |
Carotid artery aneurysm | Fusiform or saccular dilation of the carotid artery with attenuation values similar to the aorta before and after contrast administration. Curvilinear calcification of the dilated vascular wall is common. A thrombus may be seen. An intimal flap indicates dissecting aneurysm. | Common conditions associated with a neurysms of the extracranial carotid artery include a therosclerosis, congenital defects (Ehlers–Danlos, Marfan, Kawasaki, and Maffucci syndromes), trauma, and infection (mycotic aneurysm). |
Hemorrhage and hematoma | Well to poorly defined, often inhomogeneous mass lesion that may displace the adjacent structures. CT densities range from 80 HU (acute) to 20 HU (chronic). Rarely, a fluid–fluid level is evident, caused by the setting of cellular elements within the hematoma and in anticoagulated patients. | Nontraumatic (spontaneous) and traumatic etiologies. |
Benign neoplasms | ||
Carotid body paraganglioma | Sharply defined, ovoid mass, isodense to adjacent muscles, with mass center in the crotch of the common carotid bifurcation and superior extension. The tumor is intensely and homogeneously enhancing after IV bolus injection of contrast material and shows incorporation of carotid branches into the tumor density and characteristically splaying internal and external carotid artery, unlike other hypervascular lesions, such as metastases from renal cell carcinoma, thyroid cancer, and melanoma, or amyloid deposition. | In the infrahyoid neck, paragangliomas most commonly arise from paraganglia in carotid body, which is situated in the carotid bifurcation (60%–67% of all paragangliomas). Carotid body paragangliomas may affect all age groups but typically present as slow-growing, pulsatile, painless mass below the angle of the mandible, in women, in the fourth decade of life, and may be multiple in as many as 30% of patients with a positive family history of paraganglioma. Twenty percent have vagal and/or hypoglossal neuropathy. Most display no functional activity (paroxysmal hypertension, palpitations, and flushing from catecholamine secretion). Living in areas of high altitude predisposes to the formation of carotid body tumors. Paragangliomas have a tendency to occur multifocally. They can be associated with medullary carcinoma of the thyroid, in familial MEN syndromes, neurofibromatosis, and multiple mucocutaneous neuromas. As many as 10% of all carotid body paragangliomas are malignant. |
Schwannoma | Usually well-circumscribed, ovoid to fusiform homogeneous soft tissue mass, isodense or, rarely, hypodense to adjacent muscles with variable, often intense contrast enhancement. Large tumors may undergo cystic degeneration and therefore present with central nonenhancing and peripheral enhancing areas. Calcifications are exceptional. In infrahyoid neck, carotid space schwannomas typically grow between the common carotid artery and the internal vein, tend to separate the vessels, and displace the common carotid artery anteromedially, the internal jugular vein posterolaterally, the anterior scalene muscle posteriorly, the sternocleidomastoid muscle anteriorly, the visceral space to the contralateral neck, and the posterior cervical space posterolaterally. | Only 13% of schwannomas occur in the extracranial head and neck along the sympathetic chain, brachial plexus, vagus nerve, and cervical nerve roots. In infrahyoid carotid space schwannoma, patients present with an asymptomatic palpable infrahyoid anterolateral neck mass. There is a male predominance, age range from 18 to 63 y. May be multiple in neurofibromatosis type 2. |
Neurofibroma | Sporadic neurofibromas may present as solitary or multiple, ovoid or fusiform, heterogeneous, low-density masses with well-circumscribed margins. Absence of contrast enhancement is conspicuous. Large lesions can encase the carotid artery. Plexiform neurofibromas may appear as more infiltrative, poorly circumscribed and marginated fluid-density lesions that often surround the carotid artery. | Infrahyoid carotid space neurofibromas arise from the cervical sympathetic chain or vagus nerve. Women in the third decade of life are most commonly affected. About 50% of all neurofibroma cases are a ssociated with neurofibromatosis type 1. Five to 10% of lesions may undergo malignant transformation. |
Primitive neuroectodermal tumor (PNET) | The CT findings are variable and include homogeneous tumors with or without calcifications or heterogeneous lesions with cystic, necrotic areas and solid portions. After injection of contrast material, intense enhancement may be seen. | Primitive neural tumors include neuroblastoma, ganglioneuroblastoma, and ganglioneuroma. Primary or metastatic neuroblastoma in the neck typically occurs in children younger than 5 y and presents with large masses that encroach on the pharynx and larynx and that may involve cranial nerves (CN) IX to XII or the sympathetic ganglia, producing dysphagia, dyspnea, and Horner syndrome. |
Malignant neoplasms | ||
Nodal metastases from squamous cell carcinoma | Metastasis is probable if the minimal axial diameter of a lymph node of the internal jugular chain (level III and IV) is > 10 mm, the nodal shape is spherical, the ratio of the maximal longitudinal nodal length to the nodal width has a value < 2, or if a grouping of three or more lymph nodes with a minimal axial diameter of 8 to 9 mm in the drainage chains of the primary tumor is present. Nodal calcification from untreated metastatic squamous cancer is uncommon. More reliable imaging findings of metastatic disease are the presence of a central area of low density with a peripheral rim or an inhomogeneous nodal texture on contrast-enhanced CT images, due to tumor cells, interstitial fluid, necrosis, or cystic cavitation. Indistinct nodal margins, an enhancing irregular and thick nodal rim with infiltration of the adjacent fat planes and invasion of adjacent structures are straightforward CT findings for extra nodal tumor extension. Obliteration of a cervical lymph node region with nodal conglomerates is an imaging feature of extensive extracapsular infiltration. | The most common neoplasms involving cervical lymph node groups are metastases from head and neck squamous cell carcinoma. The incidence of metastatic adenopathy at initial presentation varies from < 10% in glottic cancer to 90% in nasopharyngeal cancer. CT criteria for assessing nodal metastases are based on nodal size and shape, the presence of central necrosis, the presence of a localized group of nodes in an expected node-draining area for a specific primary tumor, and extranodal tumor extension. However, CT is insensitive to the presence of nonnecrotic tumor within normal-sized lymph nodes. |
Nodal metastases from systemic primary | Most metastatic neck nodes from distant sites occur in the supraclavicular area (low-level IV and V). The CT appearance may be variable, including solid nodes, necrotic nodes, hypervascular nodes with areas of hemorrhage (renal cell carcinoma, malignant melanoma), and calcified nodes (colon cancer). | Systemic malignancy sites that more commonly create cervical neck metastatic nodes are melanoma, esophagus, breast, lung, and abdomen carcinoma or unknown primary with metastases to cervical nodes. |
Nodal metastases from papillary thyroid carcinoma | The involved nodes may be homogeneous, may enhance, have small scattered calcifications, have hemorrhage within the node, appear indistinguishable from reactive adenopathy, or be completely cavitated with a low density on CT (secondary to a high intranodal concentration of thyroglobulin), mimicking the appearance of a benign cyst. | Metastatic papillary thyroid carcinoma may have a variety of appearances at imaging. Papillary thyroid carcinoma is a relatively common cause of intranodal calcification, which may also be seen in metastatic follicular and medullary thyroid cancer. |
Nodal non–Hodgkin lymphoma | In cases of non–Hodgkin lymphoma, a single dominant node with scattered surrounding smaller nodes, nodal chain, or bilateral diffuse nodal disease may be present. Involved lymph nodes range in size from 1 to 10 cm, round or oval, well-circumscribed, often with a thin nodal capsule. Nodal density is equal or less than muscle, with homogeneous minor enhancement or a thin peripheral rim enhancement. Nodal necrosis can occur, albeit less frequently than with metastatic squamous cell carcinoma. Intranodal calcification may be seen after radiation or chemo-therapy. Rarely, it may be seen prior to therapy in aggressive high-grade lymphoma. | Non–Hodgkin lymphoma is the second most common neoplasm of the head and neck region (behind squamous cell carcinoma) and presents with • Enlarging or persistent painless noncontiguous lymphadenopathy (levels II–V, superficial nodes; also in nodal sites atypical of the usual drainage routes for squamous cell carcinoma: retropharyngeal, submandibular, parotid, occipital nodes) • Frequent involvement (23%–30%) of extranodal lymphatic (Waldeyer ring) and extranodal extralymphatic sites (orbit, sinonasal cavities, deep facial spaces, mandible, thyroid gland, salivary gland, skin, and larynx) • With or without associated constitutional symptoms (night sweats, recurrent fevers, unexplained weight loss, fatigue, and pruritic skin rash) Incidence increases with age. Risk factors for non–Hodgkin lymphoma include congenital or acquired immunodeficiency, autoimmune disorders, immunosuppressive regimens, Sjögren syndrome, and Epstein–Barr virus infection. |
Nodal Hodgkin lymphoma | Classically, Hodgkin lymphoma involves a single node or nodal cluster of the internal jugular chain, uni- or bilateral, and spreads contiguously. The neck adenopathies in Hodgkin lymphoma are large (2–10 cm) and homogeneous. Nodal density is either normal or decreased, with variable, usually mild homogeneous nodal enhancement. Necrosis, seen as low-density center, is uncommon prior to treatment. Intranodal calcification may be seen after radiation or chemo-therapy. Rarely, it may be seen prior to therapy. | Imaging cannot reliably distinguish Hodgkin lymphoma and non–Hodgkin lymphoma. Hodgkin lymphoma is less common than non–Hodgkin lymphoma. Hodgkin lymphoma most commonly involves upper anterior mediastinal and cervical lymph nodes (internal jugular, spinal accessory, and transverse cervical nodal chains). Associated mediastinal adenopathy is more common with Hodgkin lymphoma and abdominal adenopathy with non–Hodgkin lymphoma. In distinction to non–Hodgkin lymphoma, Hodgkin lymphoma primarily affects lymph nodes (> 90%) and only rarely presents in extranodal sites. Hodgkin lymphoma has a bimodal age distribution, with an early peak at 20 to 24 y and a later peak at 80 to 84 y. The median age at diagnosis for patients with Hodgkin lymphoma is ~28 y as compared with 67.2 y for patients with non–Hodgkin lymphoma. Forty percent of patients have category B symptoms (fever, weight loss, and night sweats). |
Contiguous tumor extension | Because of its locally aggressive nature, nasopharyngeal cancer can penetrate the tough pharyngobasilar fascia and buccopharyngeal fascia with deep extension, loss of adjacent fat planes, and invasion into the carotid space. Carotid artery encasement may be present in advanced stages of squamous cell carcinoma of the faucial tonsil. Tumors arising from the lateral wall of the pyriform sinus tend to spread through the thyrohyoid membrane into the soft tissues of the neck and carotid space very early. | The carotid space may be invaded by a pharyngeal squamous cell carcinoma, which may spread further craniocaudally along the neurovascular structures. Extrathyroidal extension of anaplastic carcinomas with invasion of neighboring soft tissue structures and extension into the carotid space is common (26%–53%). |








Related posts:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree