
A Surgeon’s Perspective on Portal Venous Sampling (and Selective Arterial Provocative Testing) for Islet Cell Tumors
Islet cell tumors of the pancreas and peripancreatic region are rare clinical entities, with an annual incidence of five cases per one million population. Historically, pancreatic islet cells have been presumed to originate from embryonic neural crest cells. Cells of this origin have been termed APUD cells, indicating that they have a high content of amine, have the capacity for amine precursor uptake, and contain an amino acid decarboxylase. Recent evidence using embryologic cell-tracing methods opens the possibility that pancreatic islet cells actually may be of endodermal origin rather than of neural crest origin.
Islet cell tumors of the pancreas can be divided into functional and nonfunctional varieties. Most tumors recognized clinically are functional, meaning that they elaborate hormonal products into the blood, leading to a recognizable clinical syndrome. Such functional tumors are conventionally named based on the predominant clinical syndrome and the hormonal product of the tumor (Table 46–1). Selected patients with functional islet cell tumors are candidates for portal venous sampling of hormone levels or selective arterial provocative testing. Most commonly, such patients have either insulinoma or gastrinoma. In contrast to patients with functional tumors, patients who display no recognizable clinical syndrome and who have normal serum hormone levels are considered to have nonfunctional islet cell tumors. Patients with nonfunctional islet cell tumors typically present with their tumors acting as space-occupying lesions. Such tumors lead to the clinical features of jaundice or duodenal obstruction if they originate in the head of the pancreas or upper back pain if they achieve significant size in the body or tail of the pancreas. In patients with nonfunctional islet cell tumors, there is no role for portal venous sampling or selective arterial provocative testing.
 Initial Evaluation
Initial Evaluation
There are three steps in the workup of a patient with functional islet cell tumors. First, the characteristic syndrome or abnormal physiology must be recognized. Characteristic clinical syndromes are well known for insulinoma, gastrinoma (Zollinger–Ellison syndrome), vasoactive intenstinal polypeptide tumor (VIPoma); Verner-Morrison syndrome, pancreatic cholera syndrome), glucagonoma, and the exceedingly rare somatostatinoma syndrome (see Table 46–1). The second step applicable to patient management involves the detection of hormone elevation in serum by radioimmunoassay. Such hormone elevations are detected by routine venipuncture from peripheral blood. Radioimmunoassay is widely available for the measurement of insulin, gastrin, VIP, glucagon, and somatostatin. Assays are less widely available for other gastrointestinal hormones such as pancreatic polypeptide, prostaglandins, cholecystokinin and peptide YY. The third step in the management of patients with suspected functional islet cell tumors involves localization and staging of the tumor in anticipation of operative therapy. It is at this step that the use of portal venous hormone sampling or selective arterial provocative testing may be helpful in the localization of such islet cell tumors. These procedures have found utility in cases of insulinoma and gastrinoma because these are the most common islet cell tumors and because these tumors may cause symptoms while small (<1 cm in diameter) and nonvisualized by computed tomographic (CT) scan or visceral angiography. In contrast, these procedures are uncommonly used in patients with VIPoma, glucagonoma, and somatostatinoma because these patients typically present with large tumors associated with lymph node and hepatic metastases, which are easily identified by ultrasound, CT scan, and other imaging modalities.
Tumor (Syndrome) | Clinical Features | Extrapancreatic Location |
|---|---|---|
Insulinoma | Hypoglycemia | Rare |
Gastrinoma (Zollinger–Ellison) | Peptic ulcer Diarrhea Esophagitis | Frequent |
VIPoma (Verner–Morrison, Hypokalemia pancreatic cholera) | Watery diarrhea Achlorhydria/acidosis | 10% |
Glucagonoma | Hyperglycemia Dermatitis* | Rare |
Somatostatinoma | Hyperglycemia Steatorrhea Gallstones | Rare |
*The classic skin rash seen in glucagonoma is termed necrolytic migratory erythema.
 Localization and Staging
Localization and Staging
Currently, the standard initial imaging technique favored for islet cell tumor localization and staging is spiral (or helical) CT scan.1–5 The accuracy of CT in detecting the primary islet cell tumor varies widely, ranging from approximately 35 to 85%, being largely dependent on the technique of scanning and the size of the primary tumor. The accuracy of CT scan for tumor localization is improved by the use of both oral and intravenous contrast as well as with focused scanning through the pancreas at 5-mm intervals. The CT scan also is used to assess for peripancreatic lymph node and hepatic metastases.
If the CT scan fails to detect the primary tumor, the next step in radiographic assessment often involves endoscopic ultrasonography (EUS).6,7 Rosch and colleagues were able to localize correctly 32 of 39 tumors (82%) using EUS after a prior negative CT scan (Fig. 46–1). As further experience has been gained, EUS has been shown to play an important role in the localization of pancreatic islet cell tumors.8
Another technique that has gained use in the localization of primary islet cell tumors, as well as metastatic lesions, is somatostatin receptor imaging. Following early studies that used radionuclide scanning with iodine-labeled tyrosine-octreotide,9,10 additional studies have been reported.11–14 These techniques take advantage of the presence of somatostatin receptors on many islet cell tumors,15 allowing for the identification of small primary tumors as well as hepatic and extrahepatic metastases. In most series, somatostatin-receptor scintigraphy is quite accurate for detecting pancreatic tumors and nodal metastases, particularly when the lesson exceeds 1 cm in diameter.
Visceral angiography is another tool used to image islet cell tumors of the pancreas. In particular, selective visceral angiography, focusing on selective visualization of the arterial supply to the pancreas and peripancreatic regions, may identify small tumors not localized by spiral CT.16 The accuracy of angiography in detecting the primary islet cell tumor varies from 45 to 85%, being related to radiographic technique, selectivity of the contrast injection, and size and vascularity of the primary tumor.
In a minority of patients with islet cell tumors, the primary tumor will not be localized following the initial radiographic assessment using CT, EUS, octreoscan or visceral angiography. Most commonly, this situation arises in patients with insulinoma or gastrinoma. In these cases, localization of the occult tumor can be performed using selective transhepatic portal venous hormone sampling.17–20 This invasive technique is designed to demonstrate a stepup in hormone concentration at the site where the tumor drains its hormonal product into the portal venous system (Fig. 46–2). The results of portal venous hormone sampling are used to define a region of the pancreas or duodenum believed to harbor the occult tumor. The overall accuracy of this test ranges from 70 to more than 95% and is dependent on such factors as the number of portal venous samples obtained, the persistent autonomous production of the hormone by the tumor, and the careful handling of all specimens obtained.
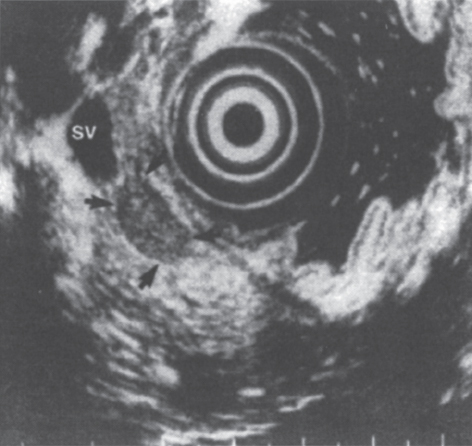
FIGURE 46–1. Endoscopic ultrasound image of an insulinoma (arrows) in the body of the pancreas. SV, splenic vein. (Reproduced from ref. 7, with permission.)
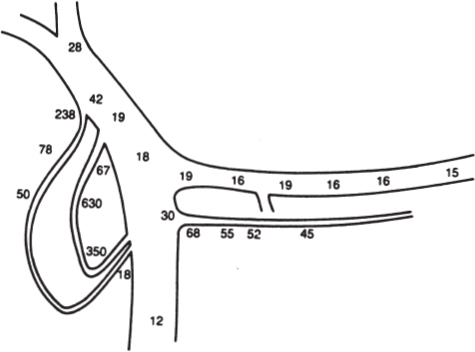
FIGURE 46–2. Schematic diagram depicting data from transhepatic portal venous insulin sampling indicating insulin levels from selective sites in μU/mL These data were correctly interpreted as showing an insulinoma in the head of the pancreas. (Reproduced from ref. 17, with permission.)
In addition to standard transhepatic portal venous hormone sampling, a newer technique has gained acceptance for the localization of occult gastrinomas and insulinomas: the selective arterial stimulation test. For gastrinoma, this technique involves selective visceral arterial secretin injection with concurrent hepatic venous sampling for gastrin.21–23 This technique takes advantage of the unique biology of gastrinoma, in that gastrinoma cells are known to respond, both in vitro and in vivo, to increased concentrations of secretin with the release of gastrin.24,25 The details of this technique involve the serial injection of 30 U of secretin through an arterial catheter into at least three separate sites: the splenic, gastroduodenal, and inferior pancreaticoduodenal arteries. Samples are drawn from the hepatic vein catheter before and immediately after the three or more arterial secretin injections. The artery that feeds the gastrinoma can be determined based on which selective secretin injection is followed by a large increase in hepatic vein gastrin concentration (Fig. 46–3). Using this technique, regional localization of the site of gastrinoma can be determined, suggesting that the gastrinoma is located either within the duodenum and the head of the pancreas or, uncommonly, in the body and tail of the pancreas. A prospective study compared transhepatic portal venous gastrin sampling to the selective arterial secretin stimulation test in 36 patients with gastrinoma.23 The results of this comparison favored the selective arterial secretin stimulation test as being more sensitive than portal venous sampling in localizing the gastrinoma, particularly small gastrinomas arising in the duodenum.
A modification of the selective arterial secretin stimulation test has been applied to the localization of occult insulinomas. This provocative test substitutes calcium for secretin and collects hepatic venous samples for insulin.26,27 Typically, a positive provocative study result is defined as observing a greater than 100% increase in hormone levels above baseline after intraarterial stimulation at either the 30- or 60-second time point. A recent study by Cohen et al26 found that provocative angiography was more sensitive than CT or octreotide scanning in a small group of nine patients with documented insulinoma or gastrinoma. In this study, provocative angiography was associated with a sensitivity of 89%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 75%.
The calcium provocation test has been shown to have an additional role in the evaluation of patients with hyperinsulinism, who may or may not have insulinoma. Pereira et al27 reported on 24 patients undergoing evaluation for hyperinsulinism, with seven of the patients having negative or equivocal CT scans, MR scans, and transabdominal US. In all seven patients, the calcium provocative test findings allowed accurate localization of the pathologic source of the insulin secretion (six solitary adenoma, one hyperplasia), followed by successful surgical intervention and postoperative euglycemia.
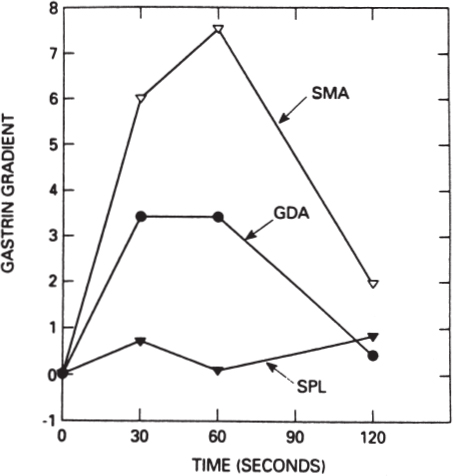
FIGURE 46–3. Graphic depiction of the results of a selective arterial secretin stimulation test. The ordinate plots the rise in hepatic vein gastrin concentration compared with basal values: 1 = 100% rise, 2 = 200% rise, and so on. SMA, superior mesenteric artery secretin injection; GDA, gastroduodenal artery secretin injection; SPL, splenic artery secretin injection. A rise in hepatic vein gastrin concentration is seen following SMA and GDA secretin injections, localizing the gastrinoma to the head of the pancreas or the duodenum. At surgery the gastrinoma was identified in the wall of the second portion of the duodenum. (Reproduced from ref. 23, with permission.)
For both occult gastrinoma and insulinoma patients, data are accumulating that favor the selective arterial stimulation test (with hepatic venous sampling) over the older technique of transhepatic portal–pancreatic venous sampling. The selective arterial stimulation test is favored because it is less invasive, less morbid, and associated with predictably high detection rates for the occult islet cell neoplasms.
 Insulinoma
Insulinoma
Insulinoma is the most common islet cell tumor. Classically, insulinoma is associated with Whipple’s triad, which includes (1) symptoms of hypoglycemia during fasting, (2) documentation of hypoglycemia with blood glucose lower than 50 mg/dL, and (3) relief of hypoglycemic symptoms following administration of exogenous glucose.28 Insulinomas are marked by the autonomous secretion of insulin, leading to spontaneous hypoglycemia with symptoms that can be classified into two groups (Table 46–2): neuroglycopenic symptoms (confusion, personality change, seizure, obtundation, and coma) and hypoglycemia-induced catecholamine-surge symptoms (diaphoresis, palpitations, tremulousness, and tachycardia). Typically, the prevention or relief of these symptoms is achieved by the consumption of carbohydrate-rich meals and snacks.
Unfortunately, Whipple’s triad is not specific for insulinoma. The differential diagnosis of adult hypoglycemia is extensive and includes reactive hypoglycemia, functional hypoglycemia associated with gastrectomy or gastroenterostomy, hypopituitarism, nonpancreatic tumors (including such entities as pleural mesothelioma, sarcoma, adrenal carcinoma, hepatocellular carcinoma, and carcinoid), extensive hepatic insufficiency, chronic adrenal insufficiency, and the surreptitious administration of insulin or ingestion of oral hypoglycemic agents. Of these entities, the most common is reactive hypoglycemia, which causes symptoms 3 to 5 hours after meals and usually is not associated with fasting hypoglycemia.




