Ajita Deodhar • John A. Kaufman
The goal of endovascular repair of an abdominal aortic aneurysm (AAA) is complete exclusion of the aneurysm sac so that it is no longer exposed to arterial pressure, thereby eliminating the risk of rupture. An endoleak is defined as persistent pressurization of the aneurysm sac post endovascular stent graft repair of the aneurysm (EVAR). This usually manifests on imaging studies as aneurysmal sac growth, but not always as sac contrast opacification. Endoleaks are reported in 4% to 30% of patients post EVAR1,2 and carry the risk of subsequent increase in aneurysm sac size and rupture.1,2
Endoleaks may be early (within 30 days post procedure) or late (after 30 days), simple with only inflow, or complex with inflow and outflow. Endoleaks are classified into five types (Table 45.1). A type I endoleak originates at the stent graft seal site in the proximal aorta (IA), distal aorta (IB), or at the iliac occluder site in an aorto-uni-iliac graft (IC). Incomplete apposition of the stent graft with the aortic wall leads to direct communication between the aneurysm sac and the systemic arterial circulation. This type of endoleak is usually seen in the early postprocedural period. However, progressive remodeling of the aorta and aneurysm sac can lead to dilation of the aneurysm neck and iliac angulation, thereby affecting the longitudinal and lateral stability of the stent graft within the aorta.3 This can lead to delayed device migration, kinking (type I endoleak), or component separation (type III). Factors that predispose to early type IA endoleak include a short, angulated, and thrombus-containing neck. Similarly, tortuous and dilated iliac arteries can predispose to an early type IB endoleak.
Type II endoleaks are the most common type of endoleaks encountered after an EVAR, accounting for approximately 40% of all endoleaks.4 In this, there is retrograde flow through one or multiple aortic branches into the aneurysm sac. The inferior mesenteric artery, lumbar arteries, and median sacral and gonadal arteries are the usual culprits. Conceptually, most type II endoleaks are like arteriovenous malformations, with multiple inflow vessels that coalesce in a nidus (the aneurysm sac) and multiple outflow vessels.
Type III endoleaks occur with structural stent graft failure. This includes junctional separation of modular devices or disruption of the graft fabric. Type III endoleaks are fairly unusual, particularly in the early postprocedure setting. Component separation can result from the previously described aortic remodeling.
Type IV endoleaks are uncommon with current endograft technologies. These endoleaks, related to graft porosity, are usually seen during the immediate post stent graft deployment and resolve spontaneously within 48 hours.
Type V endoleak is enlargement of the AAA sac without identification of a distinct endoleak. With older devices that are now no longer in use (i.e., the first-generation Excluder; W. L. Gore & Associates, Flagstaff, Arizona), sac enlargement was due to ultrafiltration of blood by the graft material. This was not a true leak. With the newer devices, a type V endoleak is most likely due to an unidentified type I through III endoleak.
DIAGNOSIS OF ENDOLEAK
Routine surveillance of patients undergoing EVAR is essential. Surveillance modalities include radiography, ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI). Endoleaks can also be diagnosed definitively on angiograms, but the invasive nature of the procedure precludes this from being a routine surveillance modality. In general, CT angiography (CTA) is the accepted standard of care.5 Hence, most patients undergoing EVAR undergo CTA at 1 month, 6 months, and 12 months followed by yearly examinations thereafter, provided there are no complications. Follow-up strategies are in evolution with a tendency toward decreased use of CT. The stent material (stainless steel, nitinol, or Elgiloy), strut thickness, and geometry can affect the choice of follow-up imaging modality.5 Patients with a nitinol endoprosthesis can undergo magnetic resonance angiography (MRA).6 Abdominal US and radiography in combination are useful in patients who are unable to get a CT or magnetic resonance examination, usually due to high serum creatinine/low glomerular filtration rate. Abdominal radiographs are helpful in evaluating for stent graft migration, separation of modular components, or limb kinking. Color Doppler US can evaluate for aneurysm sac size as well as the presence of endoleaks, although the sensitivity is operator dependent and varies from 42% to 97%.7 Recent use of second-generation US contrast agents has improved the sensitivity,8 but these agents are not widely used.
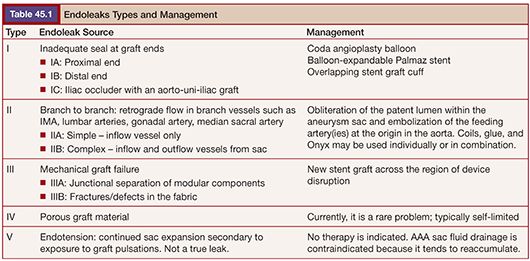
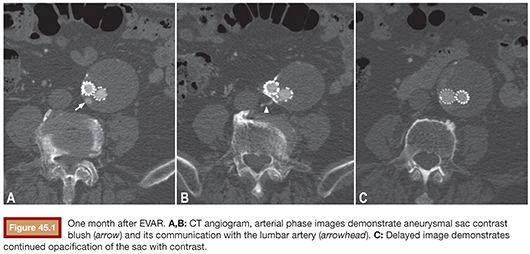
CTA and MRA demonstrate endoleaks as contrast material within the aneurysm sac (Fig. 45.1). It is important to obtain delayed-phase images as an endoleak may not be visible on the arterial phase. On US, endoleaks manifest as Doppler signal within the aneurysm sac or to-and-fro waveform in the feeding vessel (Fig. 45.2).
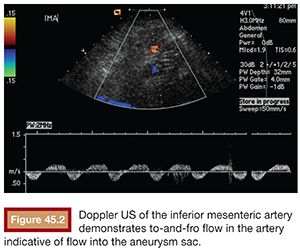
Type I endoleaks are usually evident on the post stent graft deployment angiogram as continued sac opacification of contrast resulting from insufficient proximal or distal seal (Fig. 45.3). On CTA/MRA, they will be visualized as contrast adjacent to or in communication with the proximal or distal seal zone. With a type II endoleak, contrast is visualized within the aneurysm sac, usually at its periphery with retrograde opacification of the vessel responsible for the endoleak (Fig. 45.1). The location of the contrast suggests the vessel of origin: anterior opacification may be from the inferior mesenteric artery or a gonadal artery; posterior opacification may be from lumbar or median sacral arteries. Diagnostic angiograms typically demonstrate retrograde flow, filling the culprit vessel and opacifying the aneurysm sac (Fig. 45.4). The most common sources are collateral flow to the inferior mesenteric artery via an arc of Riolan (from the superior mesenteric artery), lumbar artery via the iliolumbar branch of the internal iliac artery, or the median sacral artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








