Abdominal Aortic Aneurysm
Aneurysm of the abdominal aorta is a relatively common disease in patients over 50 years of age.1–3 Rupture of an abdominal aortic aneurysm (AAA) has a high mortality and causes as many as 30,000 deaths per year in the United States, which is more than AIDS or prostate cancer.4 It is one of the least-known killers in American society.4
More than 100 years ago, William Osler said, “There is no disease more conducive to clinical humility than aneurysm of the aorta.” This remains true today, as the diagnosis of ruptured AAA continues to confound clinicians. Misdiagnosis of ruptured AAA is common because many patients have not had a previously asymptomatic AAA formally diagnosed; they also may present with nonspecific complaints and have normal vital signs.5–7 Mortality due to AAA is decreased if the diagnosis is made prior to rupture or if the diagnosis is made rapidly after rupture of AAA.8–13
The availability of point-of-care ultrasound in the ED has allowed emergency physicians to change their approach to patients at risk for a ruptured AAA. A screening point-of-care ultrasound examination can now be obtained on patients over 50 years of age who present with pain in the abdomen, back, flank, or groin, and on those who present with dizziness, syncope, unexplained hypotension, or cardiac arrest.2,14 This practice is analogous to the immediate acquisition of an ECG for all patients with possible myocardial infarction. In this capacity, ultrasound clearly can save lives, making this application one of the indisputable benefits of emergency point-of-care ultrasound.8,9,13,15
 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS
AAA occurs in 2–5% of the population over 50 years of age and about 10% of men over 65 years of age who have risk factors for vascular disease.16–22 The prevalence is even higher in patients with first-degree relatives who have an AAA and those with peripheral vascular disease.4,23 AAA is about four times more prevalent in men than in women.24–26 The prevalence has been steadily rising in both men and women over the past several decades, so despite advances in diagnostic imaging and surgical techniques there has been essentially no change in the number of patients presenting with ruptured AAA.27–31
The risk of AAA rupture is directly related to the largest diameter of the aneurysm and increases dramatically in those >5 cm. Estimates of rupture risk are as follows: <2% per year for aneurysms <4 cm, 1–5% per year for those 4–5 cm, 3–15% per year for those 5–6 cm, 10–20% per year for those 6–7 cm, and 20–50% per year for those >7 cm.32–37 Other factors such as continued smoking, uncontrolled hypertension, and emphysema increase the risk of rupture.38,39 Also, women have a higher risk for rupture than men with the same-size aneurysm.32,40–42 Current guidelines for elective treatment of AAA suggest operative repair of aneurysms 5.5 cm or larger in the “average” patient.43 Of course, each patient’s situation is unique and requires an analysis of the risks and benefits prior to elective surgery.4
Most patients with an AAA are asymptomatic until rupture occurs. The overall mortality for ruptured AAA is roughly 80%. About 60% die prior to receiving any medical care and 40–50% of those who have emergent operative repair die.18,44–49 Rapid diagnosis and early surgical management have been shown to decrease mortality.10–13 Unfortunately, 30–60% of patients with a ruptured AAA may be initially misdiagnosed.6,44,50,51 Delayed or misdiagnosis occurs because the symptoms may mimic other common conditions such as renal colic, musculoskeletal back pain, diverticulitis, GI hemorrhage, sepsis, or acute coronary syndrome. Patients do not usually know that they have an aneurysm, and many have normal vital signs and initially appear well.6,18,47,51–53
Emergency physicians routinely manage a large number of patients over 50 years of age who have symptoms that could be consistent with a ruptured AAA. Most of these patients do not have a ruptured AAA, but those who do must be diagnosed rapidly. Even when an aneurysm is suspected, physical examination findings are not reliable for excluding or confirming the diagnosis.6,18,47,51–55 Therefore, clinicians need an accurate diagnostic screening test that can be effectively used on a large number of patients. The diagnostic test must be rapid and easy to obtain so that clinicians will have a low threshold for using it. The only diagnostic test that fits this description is point-of-care ultrasound performed by the clinician who is already caring for the patient.50 Laboratory tests are unhelpful and plain radiographs are unreliable; the two essentially have no place in the diagnostic evaluation for an acute AAA. Computed tomography (CT) is very accurate for detecting AAA but it exposes patients to ionizing radiation, is very expensive, and is not practical in unstable patients. The use of point-of-care ultrasound performed by clinicians has been extensively studied and is nearly 100% accurate for detecting or excluding AAA.5,7,56–59
In 1989, the first report on the use of point-of-care ultrasound by emergency physicians to rapidly diagnose ruptured AAA was published.60 Two prospective studies reported that emergency physicians with limited training could diagnose AAA with sensitivity and specificity approaching 100%.57,61 In 2000, a study analyzed 68 patients over 50 years of age presenting to an ED with symptoms worrisome for a ruptured AAA.56 Point-of-care ultrasound was performed by emergency physicians who had attended a 3-day point-of-care ultrasound training course but had no prior experience performing or interpreting sonograms. They detected 26 AAAs and had 100% sensitivity and 100% specificity. They concluded that relatively inexperienced providers can perform aortic ultrasound examinations accurately. In 2003, a prospective study analyzed 114 patients presenting to an ED with symptoms suggestive of ruptured AAA.7 Point-of-care ultrasound examinations were performed by emergency physicians and senior emergency medicine residents. The investigators diagnosed AAA in 29 patients, had 100% sensitivity, and 98% specificity compared with confirmatory testing, and concluded that emergency physicians could use point-of-care ultrasound to diagnose or exclude AAA. A 2005 study evaluated 238 patients who presented to an ED with symptoms concerning for ruptured AAA.5 Ultrasound examinations were performed by senior emergency medicine residents who had been trained following guidelines of the American College of Emergency Physicians.62 They found 36 ruptured AAAs and had 100% sensitivity for making the diagnosis, although their measurements were not perfect. They correctly measured aneurysm diameter in 34 of 36 patients and incorrectly measured 2 aneurysms, but recognized that the aorta was abnormal, leading to the correct diagnosis in those cases. The investigators concluded that appropriately trained emergency medicine residents can accurately determine the presence of AAA.
A 1998 study conducted by Plummer and colleagues dramatically demonstrated the need for emergency point-of-care ultrasound.13 They reviewed the medical records of 50 consecutive patients presenting to their ED with a ruptured AAA. Twenty-five patients who had an immediate point-of-care ultrasound examination had an average time to diagnosis of 5.4 minutes and a median time to disposition for operative intervention of 12 minutes. Twenty-five patients who did not receive an immediate point-of-care ultrasound examination had an average time to diagnosis of 83 minutes and a median time to disposition for operative intervention of 90 minutes. Their most important finding was that those patients who received an early point-of-care ultrasound examination had a 40% mortality compared with a 72% mortality for those patients who did not receive a point-of-care ultrasound examination.
Although ultrasound is operator dependent and the quality of the examination may be influenced by the expertise of the clinician as well as patient body habitus, point-of-care aortic ultrasound can achieve 100% accuracy with brief training.56,63–66 There is not much inter-observer variability, even in inexperienced hands, though more experienced clinicians tend to be more accurate.64,67 Training in point-of-care ultrasound of the abdominal aorta to evaluate for AAA is now a standard part of the core point-of-care ultrasound curriculum across all US emergency medicine residency programs.68
 CLINICAL INDICATIONS
CLINICAL INDICATIONS
The main indication for point-of-care aortic ultrasound examination is to rapidly identify patients with an AAA (Figure 9-1). Patients with signs or symptoms that could be attributable to a ruptured AAA should undergo an immediate point-of-care ultrasound examination. Patients with a ruptured AAA have widely varying presentations. Reasonable indications for bedside aortic ultrasound examination include all patients over 50 years of age with the following signs or symptoms:
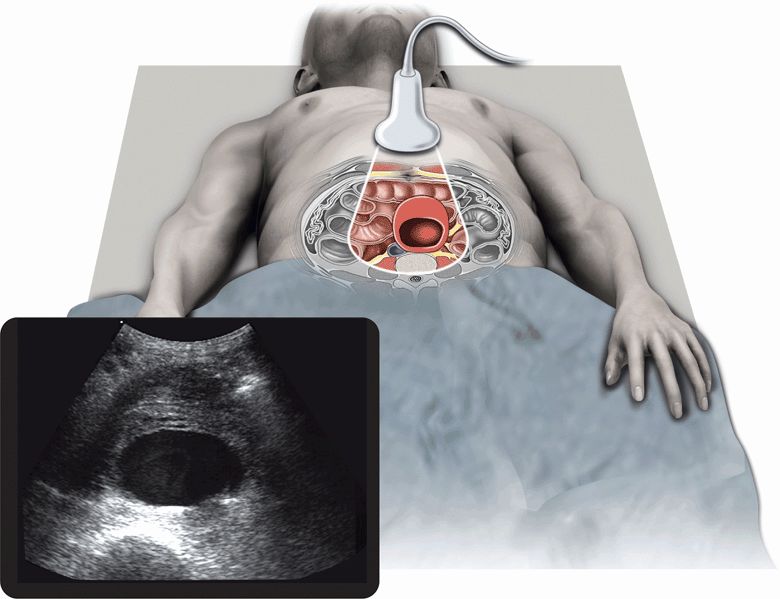
Figure 9-1. Abdominal aortic aneurysm. Ultrasound techniques and findings are outlined in the corresponding sections of this chapter.
 The classic presentation of ruptured AAA
The classic presentation of ruptured AAA
 Any pain consistent with ruptured AAA
Any pain consistent with ruptured AAA
 Unexplained hypotension, dizziness, or syncope
Unexplained hypotension, dizziness, or syncope
 Cardiac arrest
Cardiac arrest
It is certainly preferable to diagnose AAA prior to rupture since early recognition and repair carries a much lower mortality. In 2005, the US Preventive Services Task Force published recommendations that endorsed the one-time screening for AAA by ultrasonography in men aged 65–75 who had any history of tobacco use. It has been demonstrated that screening for AAA and surgical repair of large AAAs (5.5 cm or more) in men aged 65–75 with a history of tobacco use (current or former smokers) lead to decreased AAA-specific mortality.8
CLASSIC PRESENTATION OF RUPTURED AAA
The “classic” presentation of a ruptured AAA is the triad of abdominal, back, or flank pain; a palpable abdominal mass; and hypotension. This “classic” presentation is found in <25% of cases.6,47,50,51,53 Pain is the most consistent part of the triad and is present in >80% of those seeking medical attention.6,51,53 Palpation of an abdominal mass on physical examination is an unreliable finding. One study reported that a palpable mass was noted in only 18% of 329 patients presenting with a ruptured AAA.53 In addition, many thin patients with an apparent pulsatile abdominal mass have a normal abdominal aorta.54 Finally, hypotension is not universally present with ruptured AAA. Most aneurysms rupture into the retroperitoneum, resulting in a transient tamponade effect; thus, 30–50% of patients have a normal blood pressure at the time of presentation with ruptured AAA.6,18,51–53,69
PAIN CONSISTENT WITH RUPTURED AAA
Pain is the most common reason for patients with a ruptured AAA to seek medical care. Most patients complain of pain in the abdomen, back, or flank. Studies have found that about 80% of ruptured AAA patients have abdominal pain, about 60% have back or flank pain, and 22% have groin pain at the time of presentation.6,51 In another study of 329 cases of ruptured AAA, it was found that 49% had abdominal pain and 36% had back pain at the time of presentation.53 Clinicians should be aware that patients often present with referred pain to the scrotum, buttocks, thighs, shoulders, chest, or other locations.2 Many patients with ruptured AAA are misdiagnosed with renal colic, diverticulitis, or musculoskeletal pain.50 When patients present with nonspecific pain and stable vital signs, it is common to miss or delay the diagnosis. In these difficult patients, it is imperative to rapidly diagnose a ruptured AAA because those who develop hypotension prior to surgery have a higher mortality.10,13,18,70 No patient with a known or suspected AAA rupture should be considered stable regardless of initial vital signs.52
UNEXPLAINED HYPOTENSION, DIZZINESS, OR SYNCOPE
Hypotension is present in 50% or more of patients presenting with ruptured AAA.6,18,51–53,69 Altered mental status secondary to hypotension can make it difficult to obtain a history of abdominal, back, or flank pain. Therefore, patients may present with unexplained hypotension and no other clues of a ruptured AAA. Many of these patients are misdiagnosed with GI hemorrhage, sepsis, or myocardial infarction.6,50,51,53 Both hypotension and altered mental status prior to surgery are independent predictors of mortality from a ruptured AAA.18,70,71 Multiple protocols for screening ED patients who present with undifferentiated hypotension have been developed.72 One study used an empiric ultrasound examination to screen for pericardial effusion, free intraperitoneal fluid, and AAA. Another study used a similar approach that included additional ultrasound windows to evaluate the inferior vena cava (IVC), perisplenic region, and pelvis.73 Both studies pointed out that empiric abdominal ultrasonography is especially valuable when clinical history is limited or unknown and suggested that it should be a routine part of evaluation of patients with unexplained hypotension.
Relative hypotension, orthostatic hypotension, dizziness, or syncope may be precursors to overt hypotension and are indications for ultrasound examination of the abdominal aorta. Relative hypotension occurs in patients with baseline hypertension who have a decrease in blood pressure level but still have values that are within the “normal” range. These patients may present with altered mental status, vague weakness, dizziness, or syncope. A study that reviewed the records of 23 patients with a ruptured AAA found that 61% were initially misdiagnosed.51 Only 13% of patients had overt hypotension (systolic blood pressure <90 mm Hg) but 26% had syncope and 48% had an initial systolic blood pressure <110 mm Hg or orthostatic hypotension.
CARDIAC ARREST
Cardiac arrest is a fairly common presentation in patients with ruptured AAA.18 Clinicians should recognize that patients with pulseless electrical activity may be in a state of severe hypotension that could possibly be reversible if the cause is rapidly identified and aggressively treated.74 A case report described a patient with pulseless electrical activity in whom emergency physicians used point-of-care ultrasound to immediately identify mechanical cardiac activity despite lack of pulses.75 This finding led to aggressive resuscitation and a search for potential hemorrhage. They then used point-of-care ultrasound to detect a large AAA, allowing the patient to proceed directly to the operating room for surgical repair. They concluded that patients presenting with pulseless electrical activity who are found to have mechanical cardiac activity should have immediate ultrasound imaging of their aorta. Multiple approaches to rapid ultrasound exam of the pulseless electrical activity arrest victim have been described. If hypovolemia is suggested by decreased cardiac chamber volume and increased IVC collapsibility, the aorta should be promptly assessed to evaluate for possible AAA rupture.76–79
Some physicians argue that patients who have sustained cardiac arrest from a ruptured AAA have a minis-cule chance of survival and that surgical repair is a waste of resources, but this view is not supported by data.70 One study found that preoperative cardiac arrest occurred in 24% of patients with ruptured AAA, but 28% of those survived operative repair.18
SCREENING FOR AAA IN HIGH-RISK ASYMPTOMATIC PATIENTS
Ultrasound screening for AAA and its impact on mortality have been extensively studied.16,80–84 A 2005 evidence-based systematic review of population-based screening for AAA concluded that selective screening for AAA significantly improves AAA-related mortality. When the study extrapolated the findings to the entire US population of men aged 65–74 years, it estimated that 11,392 AAA deaths could be prevented over a 5-year period.9 A 2007 Cochrane review of four randomized controlled trials involving 127,891 men and 9342 women found significant decrease in mortality from AAA in men who were screened, but not for women. Only one of the analyzed trials included women, leading to the conclusion that there is insufficient evidence to determine the benefit of AAA screening in women.85
A study analyzing the cost-effectiveness of a “quick-screen” program for AAA found that the cost-effectiveness ratio (cost per life saved) was lower than the cost of breast cancer screening. A study evaluating the efficacy and cost of a large-scale screening effort in men between the ages of 65 and 75 years who had smoked at least 100 cigarettes in their lifetime found that AAA was diagnosed in 5.1% of screened patients. The cost of the exam per patient was $53.86 Properly trained emergency physicians can perform limited AAA screening examinations with 100% sensitivity.7,56 The rapidity and accuracy of an ED screening exam was reported to have an average time of 141 seconds and a mean size discrepancy of 3.9 mm between the ED exam and comprehensive imaging.87 Other trainees, such as internal medicine and surgery residents, can learn to perform accurate AAA screening examinations with little training.88,89
The Society for Vascular Surgery, the American Association of Vascular Surgery, and the Society for Vascular Medicine and Biology endorse screening of the following patient groups (based on a review of six prospective randomized trials):4,16,25,80,82,84,90–92
1. all men aged 60–85 years;
2. women aged 60–85 years with CV risk factors;
3. men and women older than 50 years with a family history of AAA.
They also recommended that patients found to have an AAA 3–4 cm in diameter have an annual follow-up ultrasound examination, those with an AAA 4–4.5 cm have a follow-up examination every 6 months, and those with an AAA >4.5 cm be referred to a vascular surgeon. While these guidelines are widely accepted and adhered to in the United States, a systematic review of seven national or international guidelines for AAA screening published in 2012 found consensus only in screening men over the age of 65 for AAA and in referring patients with AAAs >5.5 cm for surgical repair.93
 ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS
The abdominal aorta is entirely a retroperitoneal structure. It begins at the aortic hiatus of the diaphragm at approximately the 12th thoracic vertebrae and courses anterior to the spine before bifurcating into the common iliac arteries. The IVC courses to the right of the abdominal aorta and may be confused for the abdominal aorta by inexperienced operators. The psoas muscle and kidney are posterior–lateral to the abdominal aorta on the left side. The left lobe of the liver is located anterior to the proximal abdominal aorta and acts as the primary acoustic window for this area of the vessel. More caudal, the aorta is posterior to the transverse colon, the pancreas, and proximal duodenum. The distal duodenum crosses over the abdominal aorta distal to the superior mesenteric artery (SMA). Other areas of the small bowel lie anterior to the abdominal aorta as it courses distally to its bifurcation at approximately the 2nd lumbar vertebrae (or umbilicus). Components of the GI system may contain air that can block visualization of the abdominal aorta and its branches.
The abdominal aorta is 10–20 cm in length in adults with a maximum external diameter that is normally <3.0 cm (2.1 cm for men over 55 years and 1.8 cm for women over 55 years). It tapers to approximately 1.5 cm at the bifurcation, but it can be <1.0 cm in diameter in smaller adults. The first large branch of the abdominal aorta is the celiac trunk (Figure 9-2). It comes off the anterior wall of the abdominal aorta approximately 1–2 cm below the level of the diaphragm and courses anteriorly for 1–2 cm before splitting into the common hepatic and splenic arteries. The splenic artery courses to the left and follows the superior border of the pancreas before entering the spleen. The common hepatic artery courses to the right and supplies blood to the liver, stomach, pancreas, and duodenum. The SMA arises from the anterior wall of the aorta approximately 1–2 cm distal from the celiac trunk and courses caudally to supply blood to the small bowel. Both renal arteries come off the lateral wall of the abdominal aorta, just distal to the SMA; the right renal artery courses under the IVC. The paired gonadal arteries (testicular and ovarian) come off the anterior wall distal to the renal vessels. The inferior mesenteric artery comes off the anterior wall 2–3 cm proximal to the iliac bifurcation and supplies blood to the lower GI tract. Both the gonadal vessels and inferior mesenteric artery are usually difficult to visualize by ultrasound and rarely contribute to evaluation and management of the patient.
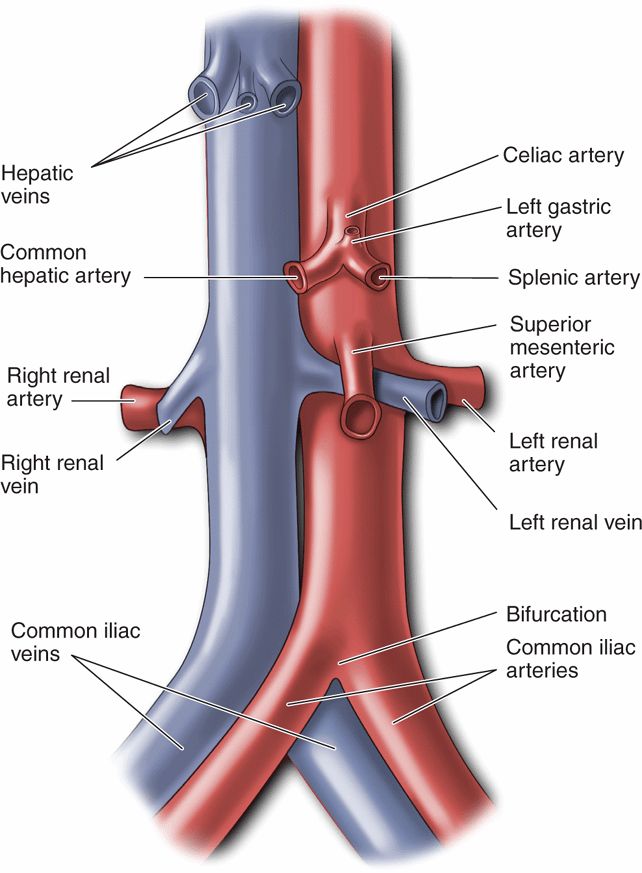
Figure 9-2. Branches of the abdominal aorta and inferior vena cava.
 GETTING STARTED
GETTING STARTED
The bedside scenario when performing an ultrasound examination of the aorta can vary from a calm physician’s office to a resuscitation bay crowded with medical personnel working together to provide simultaneous components of patient care. There are a variety of protocols that clinicians may adopt to complete an examination; however, an abbreviated examination of the aorta is usually easy to complete in <1 or 2 minutes. Ideally, evaluate the entire length of the abdominal aorta from the diaphragm to the bifurcation in both the longitudinal and transverse planes.
Examine most patients in the supine position; however, place the patient into the right or left decubital positions if excessive bowel gas is present. Bowel gas is the most common cause for inability to view the abdominal aorta; by placing the patient in either decubitus position, bowel gas may be displaced from the aorta.
Since there are no ribs or other bony structures overlying the abdominal aorta, a variety of transducers can be used for the examination, ranging from curvilinear transducers with large footprints to small phased array transducers typically designed for echocardiography. Most examiners use abdominal “presets” for the examination. Focal zones and frequency may need to be augmented to accommodate the patient’s body habitus.
Situate the machine at the patient’s right side. Place a liberal amount of gel in the area from the xiphoid process to the umbilicus. By convention, scan the abdominal aorta in the longitudinal (sagittal) plane by pointing the transducer marker dot toward the patient’s head and in the transverse (axial) plane by pointing the transducer marker dot toward the patient’s right side. The vast majority of imaging of the abdominal aorta is performed on the anterior surface along the patient’s midline (Figure 9-3). The critical portion of the abdominal aorta examination (just above the bifurcation) is accomplished in the transverse orientation with the transducer indicator pointed toward the patient’s right side.
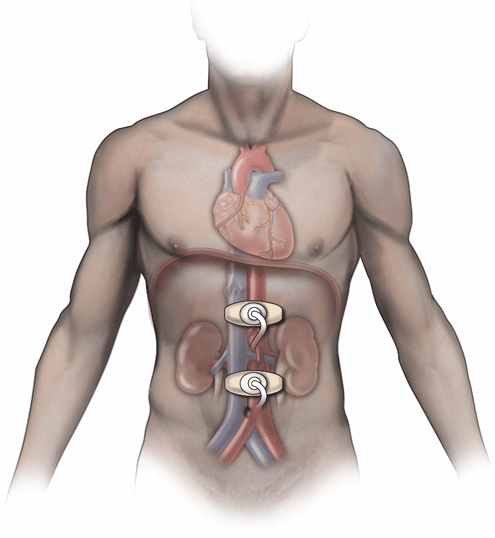
Figure 9-3. Ultrasound transducer positions for imaging the abdominal aorta. The length of the aorta can be imaged using sweeping views from these transducer positions. This can avoid potential interference from gas in the transverse colon.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 CLINICAL CONSIDERATIONS
CLINICAL CONSIDERATIONS CLINICAL INDICATIONS
CLINICAL INDICATIONS ANATOMICAL CONSIDERATIONS
ANATOMICAL CONSIDERATIONS GETTING STARTED
GETTING STARTED TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS COMMON ABNORMALITIES
COMMON ABNORMALITIES COMMON VARIANTS AND OTHER ABNORMALITIES
COMMON VARIANTS AND OTHER ABNORMALITIES PITFALLS
PITFALLS CASE STUDIES
CASE STUDIES