Abdominal Wall Disorders
Teresa Chapman, MD
LEARNING OBJECTIVES
1. Discuss implications for morbidity and mortality associated with a diagnosis of omphalocele versus gastroschisis.
2. Name the type and size of abdominal wall defect most likely to be associated with chromosomal aneuploidy.
3. Indicate appropriate imaging study for an infant with a history of gastroschisis presenting with suspected bowel obstruction.
4. Recognize features of cloacal exstrophy on prenatal imaging.
5. Identify pubic diastasis on radiography.
6. Recommend appropriate follow-up for a newly diagnosed urachal remnant.
7. List imaging features of prune belly syndrome.
INTRODUCTION
A wide range of midgut and hindgut anomalies may arise from defects in the anterior abdominal wall. Early in the 4th week of development, the embryo undergoes an elaborate folding process that transforms the flat germ disc into a convex-shaped threedimensional structure, with the endoderm, mesoderm, and ectoderm all meeting and fusing in the midline. The endodermal gut tube initially consists of the blind-ending cranial foregut and caudal hindgut, and the midgut remains open to the yolk sac through the vitelline duct.1 The boundaries of the foregut, midgut, and hindgut are determined by their vascular supply, which originates from derivatives of the vitelline arty: the celiac, superior mesenteric and inferior mesenteric arteries vascularize the foregut, midgut, and hindgut, respectively. As described in Chapter 12, the intestines normally migrate outside the abdominal cavity through the umbilical ring during a phase of rapid growth in the 6th week of development, and upon returning to the abdominal cavity by the 12th week of development, the bowel normally has rotated 270 degrees counterclockwise before mesenteric fixation occurs in the peritoneal cavity.1 Meanwhile, the urinary tract is partially derived from (1) the cloaca, which is the distal expansion of the hindgut and gives rise to the urinary bladder, and also from (2) the intermediate mesoderm in the dorsal body wall, which gives rise to the renal parenchyma and collecting systems.2 As these systems are developing, the abdominal wall is formed by infolding of the cranial, caudal, and two lateral embryonic folds.
In this chapter, abnormalities of the gastrointestinal and urinary tracts caused by incomplete closure of the ventral wall of the developing embryo are discussed, focusing on the role of diagnostic imaging, both prenatal and postnatal. Malformations of the abdominal wall to be covered include omphalocele, gastroschisis, bladder exstrophy, urachal remnant, and prune belly syndrome (PBS).
OMPHALOCELE
Omphalocele, also called exomphalos, results from failure of the umbilicus to close completely, allowing for abdominal contents to herniate outward through the umbilical ring. This anomaly is seen in 2 to 3 per 10,000 live births.1,3 The size of the omphalocele is quite variable and depends upon the location of the defect, which is not always limited to the umbilicus. Cranial fold defects resulting in an omphalocele may be associated with lower thoracic wall defects and congenital diaphragmatic hernia, whereas caudal fold deficits may be associated with bladder or cloacal exstrophy (to be discussed later in this chapter).4,5 The bowel and organs (often a part of the liver) that herniate externally are covered by a protective membrane composed of amnion on the outer surface and peritoneum and subserous fascia on the inner surface, with Wharton jelly between the two layers.1,4 This sac around the herniated viscera is one unique feature of this disorder not seen in gastroschisis, discussed below.4
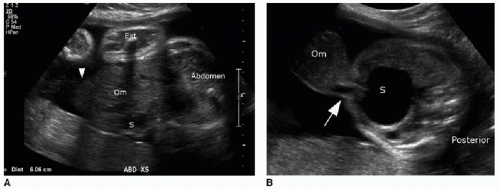
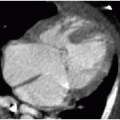
Prenatal diagnosis of an omphalocele can be made upon identification of a midline anterior abdominal wall defect allowing for herniation of visceral contents into a smoothly demarcated sac, reflecting the amnion and peritoneal sac. The umbilical
cord insertion will be at the apex of the hernia sac (Fig. 14.1).6 Because of the protective membrane, bowel dilatation and bowel wall thickening are not characteristic of this abnormality. As mentioned earlier, there is physiologic herniation of bowel into the base of the umbilical cord, which should recover usually by 11 weeks, after which time the diagnosis of omphalocele can be made.7
cord insertion will be at the apex of the hernia sac (Fig. 14.1).6 Because of the protective membrane, bowel dilatation and bowel wall thickening are not characteristic of this abnormality. As mentioned earlier, there is physiologic herniation of bowel into the base of the umbilical cord, which should recover usually by 11 weeks, after which time the diagnosis of omphalocele can be made.7
Identification of an omphalocele should prompt a search for associated anomalies. In isolated cases of omphalocele diagnosed in the first trimester, it has been observed that fetal mortality significantly increases when the mean diameter of the omphalocele is greater than 80% of the transverse abdominal diameter (Fig. 14.1A).8 A large omphalocele may contain not only bowel and liver but also stomach, spleen, urinary bladder, uterus, and ovaries.5 Even so, it is not usually the hernia itself that is the primary cause of morbidity and mortality associated with this disorder, but the associated congenital anomalies affecting 50% to 70% of these cases.4,8, 9, 10 and 11
A substantial number of affected pregnancies will be electively terminated upon prenatal diagnosis, or will spontaneously terminate in approximately one-third of cases,6,7 and therefore, the incidence of associated anomalies does decrease in live infants. A common association to consider is chromosomal aneuploidy, seen in 30% to 38% of cases, including trisomy 18, trisomy 13, trisomy 21, and 45,X.4,10 Of note, chromosomal aneuploidy is more likely to be present with a small omphalocele that does not contain liver (Fig. 14.1B).10 Congenital cardiac malformations are present in 30% to 50% of cases.4 An important syndromic association seen in approximately 6% of cases is Beckwith-Wiedemann syndrome—a hemihypertrophy disorder with an increased risk of Wilms tumor, hepatoblastoma, neuroblastoma, and rhabdomyosarcoma.12,13 This syndrome may be identified on prenatal sonography or MR imaging by macroglossia, macrosomia, polyhydramnios, and omphalocele.14
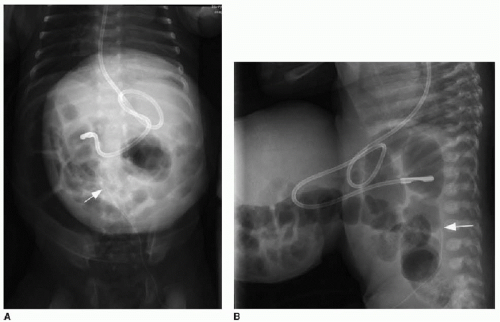
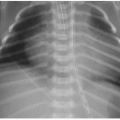
Postnatal imaging of the omphalocele itself is not necessary, although perioperative care often includes abdominal radiography to assess the bowel gas pattern. An omphalocele is appreciable on radiography as a soft tissue rounded density overlying the anterior abdomen (Fig. 14.2). Intestinal malrotation will usually be present (discussed in Chapter 12), though in some types of omphalocele, the bowel may have rotated normally.1
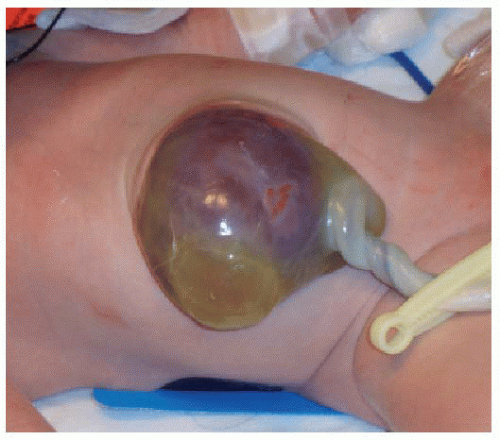
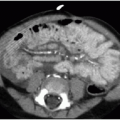
Initial treatment of a neonate with omphalocele includes gastric decompression to prevent overdistention of the herniated viscera. Careful attention is also paid to protecting the integrity of the membranous sac encompassing the abdominal organs. If the omphalocele is small enough, then a primary closure is performed, with reduction of the herniated contents and suturing of the abdominal fascia and skin. Larger omphaloceles (Fig. 14.3) require a slow secondary closure, using topical silver sulfadiazine over the membranous sac to promote epithelialization over weeks to months. Once the sac is sufficiently able to withstand pressure, elastic bandages around the abdomen are used to encourage the gradual reduction of the remaining hernia, until an elective primary closure of the abdominal wall defect can be performed.15
GASTROSCHISIS
Gastroschisis is also an anterior abdominal wall defect, but its etiology and clinical outcomes are different from those of omphalocele. In this condition, the umbilical ring closes normally and the abdominal wall defect is lateral to the umbilicus, almost always to the right.1,6 In fact, some argue that the rare left-sided gastroschisis is a separate entity entirely.16 Gastroschisis occurs in approximately 1 in 10,000 live births. The etiology of gastroschisis is controversial, with leading theories including (1) an involution of the right umbilical vein early in development, with maldevelopment of associated mesodermal elements and weakening of the abdominal wall, and (2) an arterial vasoconstrictive event that causes ischemic injury and weakening of the abdominal wall, which is supported by the observed maternal risk factors of young age,
tobacco use, and drug consumption.1,3, 4, 5 and 6,16,17 One report indicates that women younger than 20 years of age are 11 times more likely to present with gastroschisis.18 Approximately 80% to 90% of cases are isolated and are not associated with other congenital anomalies, although concomitant cardiac and genitourinary anomalies have been described.1,3, 4 and 5,9,18
tobacco use, and drug consumption.1,3, 4, 5 and 6,16,17 One report indicates that women younger than 20 years of age are 11 times more likely to present with gastroschisis.18 Approximately 80% to 90% of cases are isolated and are not associated with other congenital anomalies, although concomitant cardiac and genitourinary anomalies have been described.1,3, 4 and 5,9,18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree