Outline
Evolution of Endometrial Assessment, 835
Transvaginal Sonography, 836
Saline Infusion Sonohysterography, 836
Preprocedure Considerations, 837
Examination Technique, 837
Challenges, 837
Abnormal Uterine Bleeding, 838
Endometrial Pathology, 839
Endometrial Atrophy, 839
Endometrial Hyperplasia, 840
Endometrial Polyps, 840
Endometrial Carcinoma, 842
Tamoxifen, 842
Summary, 844
Summary of Key Points
- •
Abnormal uterine bleeding is most commonly due to dysfunctional anovulatory bleeding in premenopausal women and endometrial atrophy in postmenopausal women.
- •
Approximately 90% of endometrial cancer occurs after age 50, with the most common symptom being abnormal vaginal bleeding.
- •
Transvaginal sonography (EVS) is the initial step in the workup of postmenopausal bleeding; however, if the endometrium is not adequately visualized EVS must be followed with biopsy or additional imaging.
- •
Saline infusion sonohysterography (SIS) is a simple, low-cost, minimally invasive procedure with a high level of diagnostic accuracy.
- •
SIS is more sensitive than EVS for the detection of focal endometrial abnormalities and rivals hysteroscopy in the identification and characterization of focal lesions.
- •
SIS can be used to triage patients with abnormal uterine bleeding to the appropriate biopsy technique.
- •
Tamoxifen, a selective estrogen receptor modulator (SERM), induces endometrial proliferation, which can take the form of hyperplasia, polyp, cancer, or cystic atrophy.
Abnormal uterine bleeding accounts for up to 20% of all gynecologic visits. Once pregnancy has been excluded, the most likely cause of uterine bleeding in premenopausal women is dysfunctional anovulatory bleeding. However, as women get older, organic pathologic conditions such as endometrial polyps, submucous myomas, endometrial hyperplasia, and even frank endometrial carcinoma become more likely. The Surveillance Epidemiology and End Results (SEER) database reports that the incidence of endometrial carcinoma is 25.1/100,000 women per year. The incidence of endometrial cancer increases with age, with the median age at diagnosis being 62 years. The SEER program reports that 5.6% of new cases occur in women age 35 to 44 years, 18.4% in women age 45 to 54 years, 33.9% in women age 55 to 64 years, 23.4% in women age 65 to 74 years, and 12.6% in women age 75 to 84 years, with the remaining 6.1% at the extremes of the age spectrum. Therefore, in postmenopausal women who are not on hormonal replacement therapy, any vaginal bleeding is considered to be secondary to endometrial cancer until proved otherwise, although the incidence of malignancy in such patients ranges from 2% to 10%, depending upon risk factors.
Nonetheless, the vast majority of patients with abnormal vaginal bleeding will have either dysfunctional uterine bleeding in association with episodes of anovulation that can best be managed hormonally or expectantly with reassurance (premenopausal women), or endometrial atrophy (postmenopausal women). The utility of ultrasound to distinguish such patients from those with organic pathologic conditions in a safe, painless, and convenient manner is clear.
Evolution of Endometrial Assessment
Endometrial curettage was first described in 1843, and for many years it was the gold standard for evaluation of the endometrium, becoming the most common hospital-based operation performed on women worldwide. However, in the mid-1900s, it was realized that the technique missed endometrial lesions in approximately 10% of cases; of these, 80% were polyps. In the 1970s, vacuum-suction curettage devices allowed endometrial sampling to be performed without anesthesia in an office setting. Subsequently, cheaper, smaller, and less painful plastic catheters with their own internal pistons to generate suction became popular (e.g., Pipelle device, Unimar, Wilton, CT). They were found to have similar efficacy but better patient acceptance. Still, the Pipelle catheter was found to sample as little as 4% of the surface area of the endometrium and, therefore, had limited ability to identify malignancy confined to a polyp or localized to less than 50% of the endometrial surface. Thus, focal endometrial abnormalities such as polyps, focal hyperplasia, or carcinoma involving small areas of the uterine cavity may go undetected with undirected endometrial sampling, whether by curettage or various types of suction aspirations.
Transvaginal Sonography
Pelvic sonography is considered the first-line imaging modality of the uterus. Transabdominal imaging can be helpful in the setting of large leiomyomas or a globally enlarged uterus. However, the mainstay of endometrial evaluation is transvaginal sonography (EVS) with higher frequency transducers and, hence, better resolution. In premenopausal women, the examination should be performed early in the proliferative phase at about days 4 through 6 of the menstrual cycle. Postmenopausal women can be examined any time unless they are on cyclic hormone replacement, in which case imaging should be performed about 5 to 10 days after the last progestin tablet.
The endometrium should be evaluated in both the sagittal and transverse planes. Measurement of endometrial thickness is obtained in the midline sagittal plane perpendicular to the long axis of the endometrium at the site of maximal thickness. By convention, endometrial thickness is measured to include both the anterior and posterior layers (double layer thickness) ( Fig. 27-1 ). Fluid in the endometrial cavity should not be included in the measurement; rather, the single layer thickness of the anterior plus the posterior layer of the endometrium should be measured and summed. If the endometrium is not adequately visualized in its entirety, it should be reported as nonmeasurable and incompletely visualized. Poor visualization of the endometrium has been reported in 10% to 24% of cases and may be due to uterine position, distortion by leiomyomas or adenomyosis, or distortion of the endometrial-myometrial interface from endometrial carcinoma.

In the setting of intracavitary disease, the endometrial thickness measurement should include the lesion. A three-dimensional (3D) coronal view of the uterus has been found to be a useful adjunct to two-dimensional (2D) sonography in patients with suspected endometrial abnormalities ( Fig. 27-2 ). One study reported that additional information was obtained from the 3D coronal reconstruction in 39% of patients with an endometrial thickness of 5 mm or more. The presence and location of leiomyomas were more confidently diagnosed, in particular those in a submucosal location. Intracavitary fluid is quantified by measuring its maximal thickness in the sagittal plane.
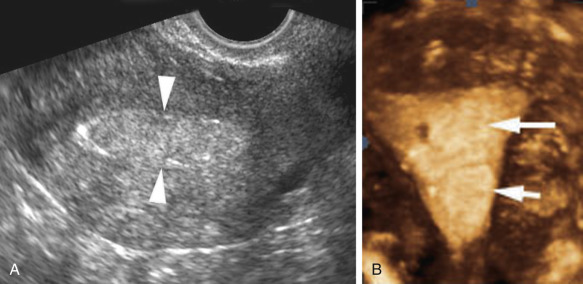
Saline Infusion Sonohysterography
Sonohysterography, hysterosonography, EVS with fluid contrast augmentation, saline-infused sonography, and saline infusion sonohysterography (SIS) are the many appellations used to describe the instillation of sterile saline into the endometrial cavity during EVS to better evaluate the endometrium and fallopian tubes. Echogenic contrast agents such as air bubbles may be used, especially when evaluating for patency of the fallopian tubes. An examination performed with particular attention to the fallopian tubes is known as sonosalpingography. SIS is a simple, low cost, minimally invasive procedure with a high level of diagnostic accuracy. Specific advantages over hysterosalpingography are the lack of ionizing radiation and the ability to more accurately characterize masses in the endometrial cavity.
Preprocedure Considerations
The SIS examination should be scheduled close to days 4 through 7 of the menstrual cycle in premenopausal women to decrease the possibility of false positive results because of the common normal irregularity and increased thickness of the secretory phase endometrium, as well as to avoid the possibility of performing the examination in a pregnant patient. However, in a patient with dysfunctional uterine bleeding it may not be possible to optimally time the examination. In these patients, a course of medroxyprogesterone acetate 10 mg daily for 10 days may be considered with the procedure timed to the withdrawal bleeding. The SIS patient should be advised to take a nonsteroidal anti-inflammatory drug (NSAID) approximately 30 minutes prior to her procedure to reduce discomfort. If this has not been done, an NSAID can be administered and the examination delayed by 30 minutes. The benefits of premedication outweigh the short delay. Prophylactic antibiotics may be prescribed if pelvic inflammatory disease is suspected or if the patient requires systemic prophylaxis for bacterial endocarditis.
Examination Technique
A calm, quietly confident demeanor and the use of familiar language without negative connotations can be very helpful in overcoming patient apprehension about the procedure. Including a female attendant in the room regardless of physician gender is recommended. Written informed consent is obtained from the patient, at which time the procedure is explained to the patient and all her questions are answered. Preparing patients for the possibility of cramping during speculum and catheter placement improves their tolerance of any discomfort.
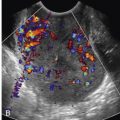
The urinary bladder should be emptied before proceeding in order to optimize patient comfort and image quality. The examination is performed with the patient in the lithotomy position with the buttocks positioned past the edge of the table. Elevation of the pelvis with a pillow or by having the patient place her hands under her hips can improve the angle of the vaginal vault, making the pelvis more accessible. Avoiding sudden movements and unnecessary noise as well as keeping instruments out of the patient’s view will help to maintain a calm atmosphere. Initial physical examination should be performed to exclude genital inflammation, determine the position of the cervix, and estimate the appropriate size and type of speculum to be used. A warm speculum is placed in the vagina to localize the cervix, which is then cleansed with antiseptic solution. Abdominal pressure on the uterus just above the pubic symphysis may help bring a posterior cervix into view. A variety of catheters may be employed to infuse fluid, but we will describe one of the easiest systems for the beginner, the dedicated balloon-tipped catheter. Before inserting the catheter, the lumen should be flushed with saline to remove all air. The catheter is then passed into the uterine cavity past the internal os ( Fig. 27-3 ) but with care not to touch the fundus, which can cause pain and elicit a vasovagal response. As much air as possible should be removed from the balloon, which is then filled with saline; otherwise posterior structures will be obscured by shadowing from air in the balloon. With experience, the balloon may be inflated within the cervix itself to decrease pain and to successfully perform studies in which one is unable to pass the catheter all the way through the cervix into the endometrial cavity. The speculum is carefully removed without dislodging the catheter. The vaginal transducer is introduced and positioned for longitudinal imaging of the uterus. The uterine cavity is distended with sterile saline under direct visualization. A slow rate of saline infusion will reduce cramping. Once adequate distention is achieved, multiplanar 2D EVS, with Doppler interrogation if necessary, as well as a 3D volume acquisition of the uterus is performed. Once the upper uterus has been satisfactorily evaluated, the balloon is deflated and additional sterile saline injected for evaluation of the lower uterine segment.

Challenges
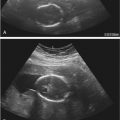
Blood in the endometrial cavity may cause confusion. Direct visualization of the cavity during gentle catheter manipulation and saline flushes can be helpful in differentiating mobile blood products ( Fig. 27-4 ) from a true endometrial abnormality. Both the stenotic and patulous cervix can be a challenge. Cervical stenosis may be encountered in a nulliparous patient, a patient who has previously had cone biopsies or loop electrosurgical excision procedure, or in perimenopausal and postmenopausal patients. A metal stylet introduced through the catheter may increase stiffness sufficiently to allow passage of the catheter into the uterine cavity. Alternatively, a small diameter sound may be used to probe the cervix, determine cervical orientation, straighten the cervix, and remove adhesions. Although not routinely used while performing SIS, a single tooth tenaculum may be used at the 12 o’clock position if the uterus is markedly flexed to put gentle traction on the cervix and straighten the uterus, allowing passage of the catheter. If a tenaculum is used, the speculum should be replaced at the conclusion of the procedure to check for cervical bleeding. The uterine cavity may be difficult to distend if fluid escapes via a patulous cervix or patent fallopian tubes. Appropriate catheter position should be confirmed, and slow injection of saline with minimal pressure may help reduce spillage from the fallopian tubes. A balloon system or 8-Fr pediatric Foley catheter should be used if the cervix is incompetent or patulous. Although there are theoretical concerns regarding the possibility of spreading adenocarcinoma into the peritoneal cavity or in causing endometriosis with SIS, neither of these considerations has proved to be relevant in practice.

Abnormal Uterine Bleeding
Postmenopausal Bleeding
Postmenopausal vaginal bleeding is extremely common and is estimated to occur in up to 55% of postmenopausal women. The Society of Radiologists in Ultrasound (SRU) defines postmenopausal bleeding as bleeding that occurs outside the expected window in women on sequential hormone replacement therapy (HRT) or any bleeding in a postmenopausal woman not on HRT. Most common are benign causes, including endometrial atrophy, polyps, hyperplasia, and uterine leiomyomas. However, approximately 7% to 14% of women with postmenopausal bleeding will have endometrial cancer (range 1-25% depending on age and risk factors; see later discussion). Women with recurrent bleeding are a particularly high-risk group. Furthermore, more than 90% of endometrial cancer occurs after age 50, with the most common symptom by far being abnormal vaginal bleeding. This statistic alone warrants evaluation of all women with postmenopausal bleeding.
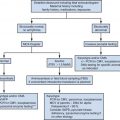
Following clinical evaluation, EVS is the initial step in the workup of a postmenopausal woman with abnormal bleeding ( Fig. 27-5 ). Ultrasound is better tolerated and more often diagnostic (>95%) compared with endometrial biopsy (EMB). The false negative rate for endometrial cancer following in-office biopsy is 5% to 15%; the rate can be reduced to 2% to 6% but only if dilation and curettage (D&C) is performed. Even with D&C, only 60% of the endometrium may be sampled and focal lesions may be missed. Up to 28% of endometrial biopsies may be nondiagnostic or insufficient. Cervical stenosis and patient intolerance may prevent EMB, and even when technically successful, an inadequate specimen may be obtained in 5% to 15% of patients. Blind biopsy is further limited by its decreased sensitivity for focal endometrial abnormalities. Although reliable for the detection of endometrial cancer occupying more than 50% of the uterine cavity, EMB can miss up to 33% of malignancies occupying a smaller area. Unfortunately, just under half of endometrial cancers have been reported to occupy more than 50% of the uterine cavity.
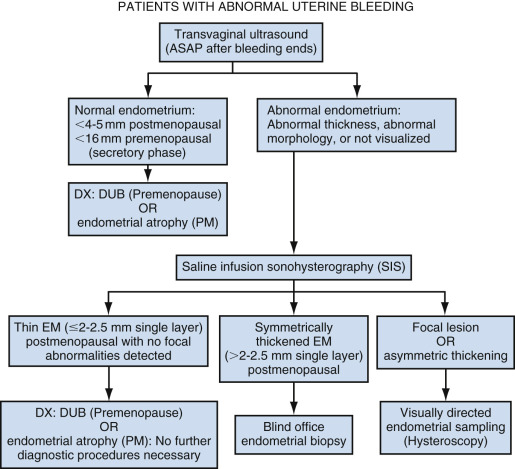
Endometrial carcinoma is rare when the endometrium is adequately visualized, thin, and homogeneous on EVS. In fact, when the endometrial thickness measures less than 4 to 5 mm on EVS, the negative predictive value for endometrial carcinoma is 99% to 100% if the endometrium is adequately visualized in its entirety. In such patients, the cause of vaginal bleeding is most likely endometrial atrophy, and no further evaluation is needed with either biopsy or additional imaging unless symptoms persist or worsen. The SRU consensus statement recommends using an endometrial thickness threshold of 5 mm or less, whereas the ACOG (American College of Obstetricians and Gynecologists) committee opinion is that the threshold should be 4 mm or less. Both societies recommend reporting the endometrium thickness on EVS as a double thickness measurement of the thickest segment of the endometrial echo complex (usually in the fundus) obtained in the midline sagittal plane and agree that further evaluation is necessary if the complex is indistinct, heterogeneous, or focally thickened. Nondiagnostic transvaginal studies occur in 2.8% to 10% of patients and are likely because of factors such as the orientation of the uterus, prior surgery, obesity, and coexisting adenomyosis or leiomyomas. Alternative evaluation should be performed if the endometrium is not visualized in its entirety, given the 15% incidence of endometrial cancer in this patient population.
Approximately 50% of cases of premenopausal and postmenopausal abnormal uterine bleeding are caused by focal abnormalities of the endometrium. SIS is more sensitive than EVS for the detection of focal endometrial abnormalities and rivals hysteroscopy in the identification and characterization of focal lesions. Furthermore, SIS can be used to triage patients with abnormal uterine bleeding to the appropriate biopsy technique. EMB or D&C is considered adequate for evaluation of a diffuse process, whereas hysteroscopy is recommended for focal abnormalities due to the risk of sampling error with EMB and D&C for focal disease as described previously. Because of the increased sensitivity of SIS for the detection of focal endometrial abnormalities, it may also be helpful in the case of discordant EVS and EMB results. One study found that 14% of patients with abnormal vaginal bleeding had abnormalities on SIS despite a normal appearance of the endometrium on EVS.
When using an endometrial thickness threshold of 5 mm, the sensitivity of EVS for endometrial cancer is 96%, with the positive predictive value increasing as the thickness of the endometrium increases. This compares with a sensitivity of greater than 85% for EMB. If the threshold for endometrial thickness is decreased to 4 mm, there is no significant change in the sensitivity of EVS for detecting endometrial cancer. However, specificity decreases and there will be more false positive diagnoses. However, this opinion was not uniformly shared by all participants at the SRU consensus panel on postmenopausal bleeding, with the dissenters recommending 4 mm as a threshold. All agree that the use of HRT increases the false positive rate at either threshold.
Although much energy has been devoted to determining the threshold endometrial thickness in a postmenopausal woman with vaginal bleeding, there is no consensus on the normal endometrial thickness in a postmenopausal woman without vaginal bleeding. However, the latter scenario is not infrequently encountered due to the widespread use of EVS for evaluation of the female pelvis. If the threshold of greater than 5 mm that is recommended by the SRU for women with postmenopausal vaginal bleeding is applied to asymptomatic women, the false positive rate and consequently the number of biopsies performed in normal women would be unacceptable. Although there is no standardized cutoff for endometrial thickness in a postmenopausal woman without vaginal bleeding, all agree it should be higher than 5 mm in this patient population with low pretest probability of endometrial cancer.
When applying a decision analysis approach to determine the endometrial thickness in asymptomatic postmenopausal women at which the risk of endometrial cancer approaches that of symptomatic postmenopausal women with vaginal bleeding and an endometrial thickness more than 5 mm, the result has been reported to be over 11 mm. An endometrial thickness over 11 mm in an asymptomatic postmenopausal woman carries an endometrial cancer risk of 6.7%, comparable to that of 7.3% in a postmenopausal woman with vaginal bleeding and an endometrial thickness greater than 5 mm. This threshold would result in biopsy of only 0.25% of women to detect 87% of occult endometrial cancers. For the more conservative endometrial thickness threshold of 4 mm recommended by ACOG for symptomatic postmenopausal women, the corresponding normal endometrial thickness in asymptomatic postmenopausal women is considered to be less than 10 mm, with an endometrial thickness more than 10 mm in an asymptomatic postmenopausal woman carrying a 5.8% risk of endometrial cancer.
EVS is not recommended as a screening tool for endometrial cancer in asymptomatic postmenopausal women, but this analysis provides some guidance on how to manage the thickened endometrium incidentally observed in a postmenopausal woman without bleeding on an ultrasound scan performed for other reasons. However, the decision to biopsy the endometrium will no doubt include overall assessment of the patient, including risk factors for endometrial cancer.
Premenopausal Bleeding
Dysfunctional uterine bleeding in a nonpregnant premenopausal woman occurs as menorrhagia, metrorrhagia, or menometrorrhagia. There is no widely accepted threshold for abnormal endometrial thickness in premenopausal women, although typically diffuse homogeneous thickening greater than 16 mm in the secretory phase is used (there is no accepted threshold value in the proliferative phase). However, a thickened endometrium in the late secretory phase of the menstrual cycle is often normal, so routine studies ideally should be performed in the early proliferative phase to minimize false positive results. The sensitivity and specificity of EVS for detecting endometrial disease in the premenopausal population using a cutoff value of more than 16 mm are not optimal at 67% and 75%, respectively. In the setting of a diffusely thickened endometrium or persistent bleeding unresponsive to hormonal therapy, SIS is indicated because of its ability to detect abnormalities in patients with normal EVS and to differentiate focal from diffuse processes, allowing triage to the appropriate biopsy technique. Focal abnormalities such as asymmetric thickening, cystic change, and the hyperechoic line sign should be further evaluated regardless of endometrial thickness and be triaged to hysteroscopy for direct visualization and biopsy. The hyperechoic line sign is indicative of a focal intracavitary process and is thought to represent the echogenic endometrium abutting the intracavitary mass or the interface between the mass and surrounding endometrium. Overall, SIS is better tolerated and more often technically successful in premenopausal women than in postmenopausal women.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree