Abscess Drainage
It is important to consider clinical status in ultrasound-guided therapeutic procedures for abscess drainage, and it is equally important to follow a standardized technique. The success rates published for ultrasound-guided abscess drainage vary widely, but a realistic range is 70 to 95%, depending on the patient groups treated.1–4 Published complication rates also vary widely,5–16 with a realistic range from <3 to 15%. A similar range is reported for mortality rates.
A complicated multilocular abscess with difficult drainage access should be managed by a combination of medical and surgical treatment. These severe cases have lower success rates and higher mortality.17,18
15.1 Historical Considerations
The drainage of body fluids has been practiced since antiquity. Available materials were quite limited, however. Drainage tubes were often fabricated from lead, which was easy to bend to the desired shape. The development of modern materials was necessary for the creation of effective drainage systems that could remain indwelling for some time. Polyethylene, for example, was discovered by the chemist Hans von Pechmann in 1898 and was first produced industrially by Reginald Gibson and Eric Fawcett in England in 1933. Other historical issues are reviewed in Chapter ▶ 2(Interventional Materials and Equipment).19,20
15.2 Preliminary Remarks, Etiology
It is important to identify underlying factors that have major prognostic significance such as underlying malignant diseases, immune defects, diabetes mellitus, or chronic inflammatory bowel diseases treated with immunosuppressant agents. Advanced age is a negative prognostic factor but does not alter the approach to management.
Percutaneous drainage has become the standard treatment for abdominal and pelvic abscesses. Its success rate is high, but there is still a recurrence rate of 5 to 10%. One retrospective analysis found that surgery was avoided in 56% of pelvic abscesses treated by percutaneous drainage. The mean time to recurrence was 51 days (2 to 365 days), and postoperative abscesses were more likely to require further surgery.3 In another study, experienced interventionalists had a 100% success rate for entering the abscess cavity and a 98% success rate for establishing catheter drainage. Again, however, not all abscesses could be successfully treated by percutaneous drainage alone; surgery was avoided in 88% of the cases.21
The differential diagnosis of infectious tropical diseases relies on a combination of serology, detection of eosinophilia, and analysis of IgE levels. The history should include information on possible trips to tropical or subtropical latitudes or endemic regions. Supplemental information should include possible underlying immunosuppression due to HIV infection, for example, or other sexually transmitted diseases.
The following factors and mechanisms may be important in the etiology and pathogenesis of abscess formation:
Primary versus secondary abscess formation
Contiguous spread
Sepsis
Postoperative or postinterventional abscess formation (after local ablative procedures, embolization, etc.)
Underlying hepatobiliary disease (mainly involving the liver)
Underlying acute or chronic inflammatory bowel disease (in which case drainage alone is less likely to be successful)
Previous trauma
Predisposition due to weakened host defenses (immunosuppression, HIV infection, diabetes mellitus, advanced age)
Idiopathic factors
15.3 Selection of Imaging Modality
Ultrasound, conventional radiographic techniques, CT, and MRI are all used as guidance modalities in the interventional treatment of abscesses. Regardless of the modality used, imaging should be contrast-enhanced and should include the whole abdomen because appendicitis with complications, peridiverticulitis, and acute or chronic inflammatory bowel disease are sometimes diagnosed in patients with indeterminate liver lesions.
15.3.1 Ultrasound
Careful attention should be given to the access route in the planning of ultrasound-guided abscess drainage. Adequate guidance requires visualization of the entire route.22 Drainage should not be done through two body cavities, and liver abscesses should never be drained through the pleural cavity. Other advantages of ultrasound guidance are the real-time display and high resolution. A potential disadvantage is the confined field of view, which limits visualization of the access route.
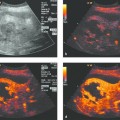
The underlying process giving rise to an abscess is a phlegmonous inflammation. A perifocal reaction is often observed in immunoreactive patients, especially after the administration of a contrast agent (this applies to contrast-enhanced ultrasound as well as contrast-enhanced radiographic methods). Contrast-enhanced ultrasound is the modality of choice for positively distinguishing a phlegmonous inflammation (▶ Fig. 15.1) from an abscess (▶ Fig. 15.2). This is due mainly to the high spatial resolution of ultrasound and the fact that the contrast agent (e.g., SonoVue) remains strictly within the intravascular compartment. The description of the abscess should include the assessment of criteria such as “internal echoes” (vascularization, perfusion) and “loculation.”
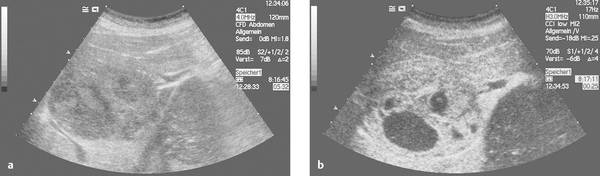
Fig. 15.1 a, b Phlegmonous inflammation with initial liquefaction (liver abscess). The inflammatory process should still be treated medically at this stage because it consists almost entirely of perfused tissue areas and dilated bile ducts (due to biliary disease). The treatment of choice in this case was endoscopic retrograde cholangiography (ERC), sphincterotomy, and stone extraction.
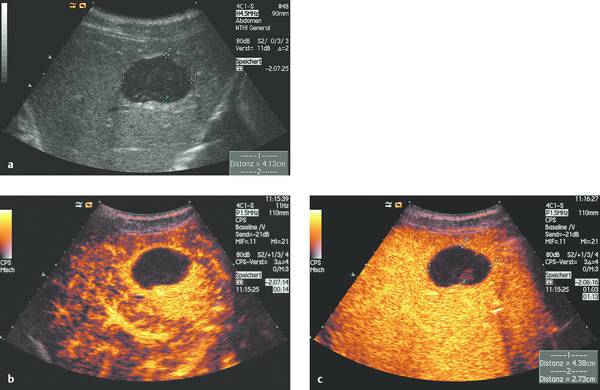
Fig. 15.2 Typical sonographic appearance of an abscess (a), which required (single) percutaneous drainage and irrigation (primary drainage). Note the ring pattern of enhancement, which is typical of an abscess (b, c).23–25
The detection of intralesional air echoes is helpful in making a diagnosis. Approximately two-thirds of liver abscesses are solitary, depending on the underlying disease, and have an average size of approximately 7 cm when first manifested and diagnosed.15,26,27
It should be noted that amoebic abscesses may be isoechoic to surrounding liver and may be identifiable as liquid masses only after contrast administration.
15.3.2 Conventional Radiographic Drainage
Conventional radiography is rarely used today for the guidance of percutaneous abscess drainage, but it may still be helpful as an adjunct to sonographic guidance.
15.3.3 Computed Tomography
Unlike ultrasound contrast agents, the contrast media administered for CT and MRI also diffuse into the interstitium. Because of this, CT and MRI cannot distinguish between perfused and nonperfused areas with the same precision as contrast-enhanced ultrasound.
15.3.4 Magnetic Resonance Imaging
MRI is rarely used as a primary study for abscess diagnosis. Abscesses generally have low signal intensity on T1-weighted images and high signal intensity on T2-weighted images due to their fluid content. Images after IV contrast administration (e.g., gadolinium-DTPA) show peripheral rim enhancement.
15.4 Devices
15.4.1 Drainage Catheters
Which Catheter?
A variety of drainage catheters are available on the market (see Chapter ▶ 2) (▶ Fig. 15.3, ▶ Fig. 15.4).
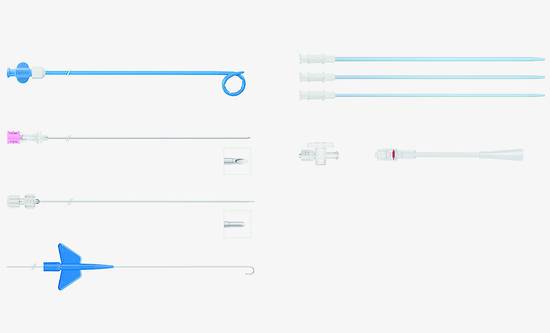
Fig. 15.3 Instrument set for the external drainage of viscous fluids by the Seldinger technique (OptiMed). Materials: OD (soft polyurethane). Components of the set: abscess drainage catheters (diameters 8–16F, lengths 30 and 40 cm). Two-part puncture needle with echogenic tip (diameter 1.3 mm, 17.5 gauge, length 20 cm). Two-part obturator (diameters 1.3–1.8 mm, lengths 33 and 43 cm). Schüller-type catheter exchange wire (diameter 0.035 inch, length 100 cm, 10-cm flexible tip, 3-mm J curve, 40-cm rigid shaft, flexible end). Dilators (diameters 6–18F, length 20 cm). Three-way stopcock. Rotating adapter, male Luer lock.
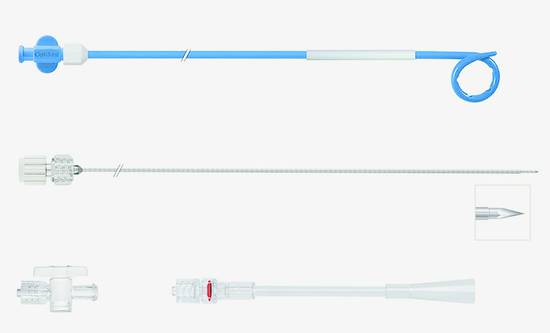
Fig. 15.4 Universal instrument set for the percutaneous drainage of viscous fluids by the trocar technique (OptiMed). Materials: OD (soft polyurethane). Components of the set: abscess drainage catheters (diameters 8–14F, lengths 30 cm). Two-part puncture needle with trocar point. Three-way stopcock. Rotating adapter, male Luer lock.
Trocar or Seldinger Technique
Most drains today are mounted on a blunt trocar when supplied. They consist (from outside to inside) of the catheter itself, made of flexible material, a trocar (a blunt steel cannula), and a sharp puncture needle. The trocar technique is ideal for short access routes that are perpendicular to the skin surface. Catheters in the size range of 10F to14F are recommended in severely ill patients and patients with extensive abscess formation. While the Seldinger technique was once preferred for larger abscesses, it is less commonly used today. The Seldinger technique is preferred in the following situations: difficult access routes, insertions tangential to the skin surface, and large-bore catheters (>14F). In muscular patients, moreover, it is often difficult to penetrate the anterior abdominal wall with a trocar.
Practice
The trocar technique (8–10F) has proven excellent for the drainage of uncomplicated abscesses in everyday practice.
Considerations on Catheter Size
Drainage catheters are supplied in standard outer diameters ranging from 6F to 16F (see Chapter ▶ 2). The choice will depend on the expected consistency of the abscess contents. Catheter diameters smaller than 8F are generally too small to provide adequate drainage and irrigation.
Practice
The typical size of an abscess drain is 10F. The exact size will depend on the etiology of the abscess, its (potentially difficult) location, viscosity, possible loculation, and the proportion of solid contents.
While small-bore catheters are usually easy to place by direct insertion, larger drains require a bolder approach. If the drain cannot be introduced easily, the tract should be dilated in small increments. This has proven helpful for catheter sizes >10F. Generally it is sufficient to dilate the tract 2F less than the catheter size. Any slight bleeding that occurs when the dilator is withdrawn should be adequately controlled by pressure from the snug-fitting drainage catheter.
Catheter Properties
Most drainage catheters have a pigtail loop at the distal end and are straightened for insertion. This means that the catheter material must have good “shape memory,” and almost all modern materials possess this property. Some manufacturers provide a locking drawstring to stabilize the distal loop.
Other crucial material properties are kink resistance and low surface friction. Because a thin wall is best for optimizing the relationship between outer diameter and inner lumen, special wall structures have been designed. An example is the Navarre universal catheter with its spiral laminated wall construction, which is resistant to kinking and compression despite the thin wall. For deeper insertions, it may be necessary to check the drain placement radiographically, so the catheter must be visible on radiographs. This may be achieved, for example, by adding barium sulfate to the catheter material. Other options are embedding a radiopaque thread in the drain wall or placing a metal ring at the distal end. In most cases, however, drain position will be checked during subsequent special studies in which contrast medium will be used; so isolated visualization of the catheter is not a crucial step. The arrangement of the drainage holes (usually 5 in number) is important in catheter selection. For abscess drainage, all the holes should be in the area of the catheter tip or pigtail. Hydrophilic catheter coatings are helpful in that they facilitate catheter advancement through the tissue. Most catheters have a conical tip with a short or long taper. This feature may adequately dilate the drainage tract, eliminating the need for separate dilators.
The pigtail drain is advanced over the cannula for direct insertion, or it may be slid onto a metal introducer for placement by the Seldinger technique so that the catheter will not distort the guidewire as it tends to reassume its coiled shape. When the catheter is slid onto the introducer, a straightening sleeve (already on the catheter shaft in most cases) is advanced to help prevent damage to the inner wall of the drainage catheter (this is described more fully in Chapter ▶ 2). Soft plastic stylets can also be used to straighten the pigtail for insertion.
15.5 Indications
Typical indications for abscess drainage are as follows:
Hematogenous abscesses in parenchymal organs
Abscesses secondary to outflow obstruction (e.g., biliary liver abscess)
Septic embolism (e.g., splenic abscess in endocarditis)
Complications of Crohn disease28–30
Circumscribed abscess due to anastomotic leakage
Confined perforation (e.g., in diverticulitis)
Posttraumatic changes
15.6 Contraindications
Percutaneous abscess drainage may be done as either an elective or an emergency procedure. While an elective drainage procedure should follow the guidelines stated above, there are life-threatening situations in which an increased peri-interventional risk must be accepted. An example would be severe sepsis with multiorgan failure.
Generally valid contraindications to percutaneous abscess drainage include the following:
Uncooperative patient
Lack of informed consent
Quick value (thromboplastin time) <50%
Platelet count <50 × 109/L
Partial thromboplastin time >50 seconds
Inability to visualize the precise abscess location
Uncertain access route along vulnerable structures
Additional limitations should be noted in cases where pharmacologic obliteration is proposed (e.g., PAIR: puncture–aspiration–injection of alcohol–reaspiration):
Cyst communicating with the biliary tract
Cysts in the central nervous system, lung, or urogenital tract
Calcified cyst (= not an indication)
15.7 Patient Preparation
Because percutaneous abscess drainage is an invasive procedure, the physician should provide the patient with a detailed explanation that includes benefits and possible complications, and informed written consent should be obtained before the procedure (Chapter ▶ 3). The patient should be informed of possible surgical alternatives, if they are available. It may be necessary to waive the informed consent requirement in emergency situations (e.g., for septic patients).
15.8 Treatment Options
15.8.1 General
Before a presumed abscess is drained, it is necessary to exclude a possible malignant condition associated with necrotic metastases (for example, larger metastases from neuroendocrine tumors may contain necrotic areas that mimic the ultrasound appearance of an abscess).31 An echinococcal etiology should also be excluded because of its different therapeutic implications. Ultrasound can reliably diagnose most echinococcal cysts but is not always adequate, and serologic testing may be required (Chapter ▶ 17).32 Tuberculosis of the liver is feared in large portions of Asia and Africa.33 The treatment of amebic abscesses is usually pharmacologic (see below).
15.8.2 Medical Treatment Options
Pharmacologic treatment strategies may vary, depending on the underlying cause, and are described under specific disease headings.
15.8.3 Surgical Treatment Options
Surgical treatments include open catheter drainage, open surgical drainage, and partial hepatectomy. Surgical treatment has proven effective for large and complex abscesses that are not accessible by other means, multilocular abscesses, and in cases with failed percutaneous treatment.34
15.9 Technique of Percutaneous Abscess Drainage
15.9.1 Preparation
Assistants
Assistants prepare for the procedure by laying out the sterile instruments and selecting the necessary drain in consultation with the physician. A highly systematic routine should be followed during the procedure itself, as it involves the drainage of infectious material that may be hazardous to both the patient and staff (▶ Fig. 15.5) (Chapter ▶ 10).
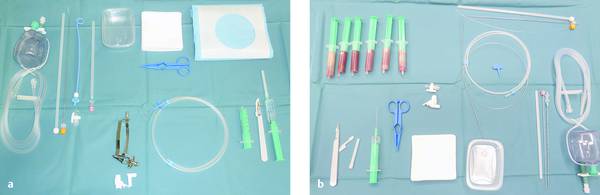
Fig. 15.5 Table setup (a) at the start and (b) at the end of the procedure with the drained abscess contents.
A nonsterile assistant should be present for passing any necessary additional materials and monitoring the patient.
Accurate Abscess Localization
Percutaneous abscess drainage always starts with accurate imaging localization of the intended target. If the abscess can be clearly visualized, the drainage route is determined, which also defines the location of the puncture site in the skin. That site is marked with a water-soluble marker.
Skin Preparation
The skin site is aseptically prepared by wiping and spraying, then covered widely with a fenestrated drape.
Sedation
Patient sedation (e.g., with midazolam) is not an absolute requirement but may be helpful in some situations (Chapter ▶ 11).
Local Anesthesia
Local anesthesia is usually performed with a 1% local anesthetic solution. The most commonly used agent is 1% lidocaine. It is important to aspirate before injecting the solution since intravascular administration could produce a systemic effect. A subcutaneous or intracutaneous wheal is placed to numb the skin. The hypodermic needle may be 22-gauge (black), 23-gauge (blue), or even 26-gauge (brown) in extremely sensitive patients. Deep analgesia can be produced with somewhat longer needles of 21-gauge (green) or 20-gauge (yellow). An alternative in very obese patients is a 20- or 22-gauge Chiba needle 220 mm long, initially using a subcutaneous needle and then changing to a longer needle (green).
15.9.2 Initial Needle Insertion
Because drainage catheters cannot be inserted directly through the skin, an initial stab incision is made with a pointed scalpel. A needle of adequate size is introduced through the incision and advanced into the abscess under ultrasound guidance (▶ Fig. 15.6).
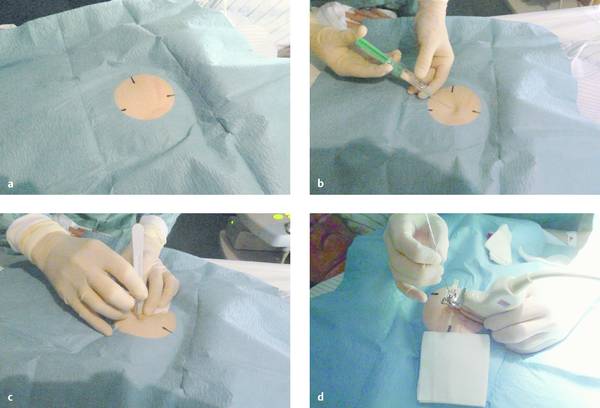
Fig. 15.6 Percutaneous needle insertion.
a Sterile draping of the skin site.
b Local anesthesia.
c Stab incision.
d Ultrasound-guided insertion of the needle into the abscess.
Often the abscess contents are aspirated in an initial step to confirm the diagnosis. This is done with a hollow needle stiffened by a stylet. Regarding needle caliber, 22-gauge needles are generally more difficult to visualize with ultrasound, while 18-gauge needles allow for immediate 0.35-inch wire guidance and can aspirate even viscous abscess contents. A 20-gauge needle (trade-off between the 18- and 22-gauge) is easy to visualize and less traumatizing than an 18-gauge needle but accommodates a smaller wire diameter.
Checking Catheter Placement
Catheter placement can be checked by conventional fluoroscopy. With adequate ultrasound visualization, we also favor sonographic localization aided by the intracavitary instillation of an ultrasound contrast agent (e.g., SonoVue). The direct injection of ultrasound contrast medium through the catheter (diluted at 1–2 drops per 20 mL saline solution) gives excellent visualization of the abscess cavity and the catheter itself, which may be poorly visualized with precontrast ultrasound due to the surface properties of the material. The contrast injection can immediately confirm (▶ Fig. 15.7) or exclude (▶ Fig. 15.8) communication of the abscess cavity with the biliary tract.
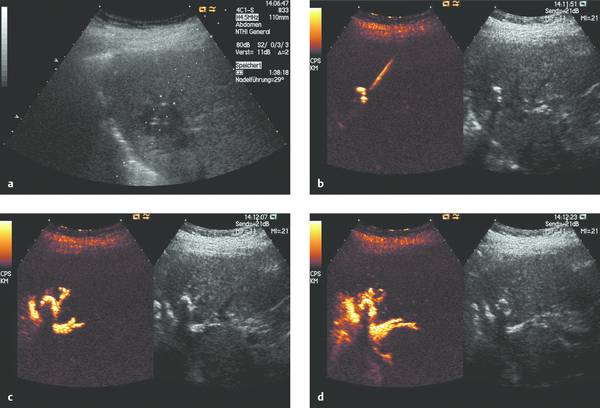
Fig. 15.7 a–d Ultrasound-guided abscess drainage using SonoVue to visualize the abscess cavity. Contrast administration confirms that the abscess communicates with the biliary tract.
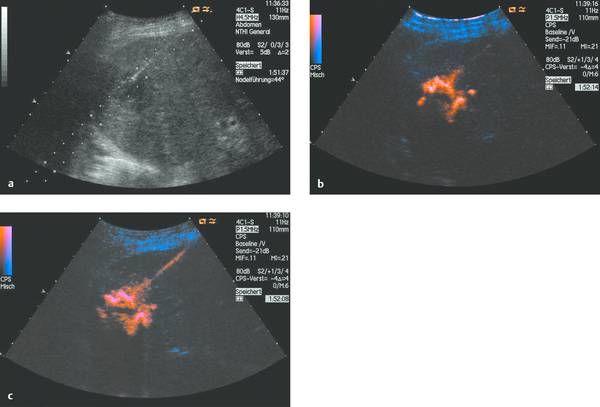
Fig. 15.8 a–c Ultrasound-guided abscess drainage using SonoVue to visualize the abscess cavity. Contrast administration shows that the abscess does not communicate with the biliary tract.
It should be noted that both intracavitary and intravenous contrast administration can be combined with real-time ultrasound guidance to confirm complete evacuation of the abscess contents (▶ Fig. 15.9).
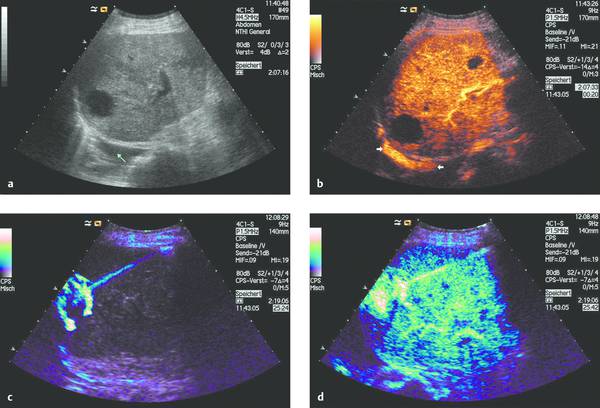
Fig. 15.9 Abscess communicating with the pleural cavity (a, b). Abscess drainage is performed under ultrasound guidance with SonoVue instilled into the abscess cavity (c) and also administered intravenously (d). The latter permits the detection (or exclusion) of any undrained portions of the abscess.
As stated earlier, two main techniques are available for catheter insertion. The trocar technique is simpler and less time-consuming, but it involves the primary insertion of a relatively rigid, large-bore instrument, making it difficult to bypass vulnerable structures. The Seldinger technique involves a more subtle initial approach in which a thin needle is passed safely and accurately to the targeted site. After placement of the guidewire, the tract is enlarged with serial dilators.
15.9.3 Trocar Technique
Once the operator has decided on the trocar technique, a two- or three-part instrument assembly is inserted directly into the abscess in one step (see Chapter ▶ 2). The procedure concludes with removal of the stylet and hollow needle. Contrast medium is then injected to confirm correct catheter placement.
Seldinger Technique
The Seldinger technique begins with the insertion of a fine needle. A 1.1-mm Chiba needle will accommodate a 0.035-inch guidewire. With a thinner needle (e.g., 0.7 mm diameter), a 0.018-inch guidewire is inserted first and the tract is carefully dilated to the size of the larger wire, which is then used for further dilation. These wires are kink-resistant and permit dilation of the needle tract using Seldinger technique. The use of a Lunderquist wire is advantageous in that the highly stable guidewire facilitates passage of the dilator, but the stiffer wire also increases the risk of perforation. Generally, however, there is no need to dilate the tract to 10F, and a drainage catheter with a tapered distal end can be advanced directly over the guidewire and into the abscess. When the guidewire is removed, the catheter reassumes its distal tip shape, which usually consists of a pigtail loop with or without a locking drawstring. Finally the catheter is secured at skin level with a retention plate or sutures (▶ Fig. 15.10).
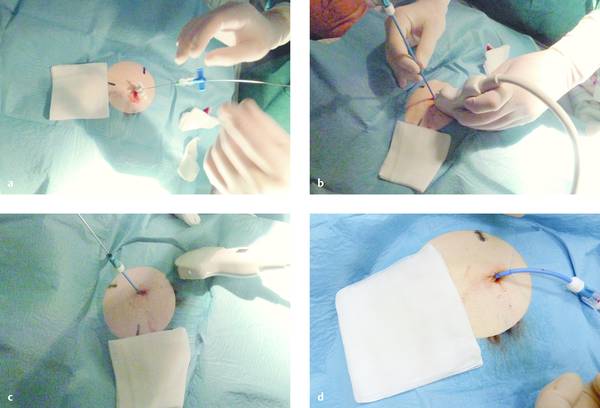
Fig. 15.10 Seldinger technique with a Chiba needle.
a The Chiba needle has been placed, and a guidewire is introduced through the needle.
b The drainage catheter is advanced over the guidewire.
c The guidewire is removed.
d The catheter is secured with sutures.
Needle Aspiration
Quantitative needle aspiration of the abscess contents (with or without irrigation) for decompression purposes is appropriate for abscesses smaller than 5 to 8 cm and is usually worth considering. It can be repeated 1 to 3 days later if necessary. Studies recommend that a drainage catheter should be placed after 2 or 3 needle aspirations if complete evacuation is not achieved. Needle aspiration achieved good results in several studies compared with drain placement for lesions up to 50 mm in size. Arguments made for percutaneous needle aspiration and against catheter drainage are that needle aspiration under ultrasound guidance is technically easier, that the abscess cavity is entered in a very high percentage of cases, and that the great majority of complications are caused by the drainage catheter rather than the initial puncture. (It should be noted that the latter statement is not accepted in general.) Thus, the decision should be made on a case-by-case basis and depends on factors that include the size, encapsulation, and location of the abscess (e.g., parenchyma abscess, interenteric abscess, retroperitoneal abscess). Some situations, such as multiple liver abscesses, may call for the use of both techniques: catheter drainage and quantitative needle aspiration.
There is a small amount of evidence suggesting that catheter drainage is better for abscesses larger than 50 mm.35 Other authors report a lower success rate for needle aspirations.36 One drawback of the published data is that different strategies were employed. The authors who reported an advantage of needle aspiration performed significantly more interventions and used thinner drains (e.g., 8F6), while authors who favored catheter drainage abandoned needle aspiration after only two attempts. Interestingly, both procedures required the same length of hospitalization.
Regardless of the drainage procedure used, close-interval imaging follow-up is essential. When a drainage catheter has been placed, success can also be assessed clinically by monitoring the drain output.
Drainage
In contrast to simple needle aspiration, catheters are used for abscess drainage. Once a drain has been placed, it is left indwelling for some time. The drain may irritate neighboring structures and create a portal for ascending infection. Before the needle is inserted, therefore, it is essential to obtain a clear image of the intended target and select the shortest possible drainage route that is still considered safe. The proposed drainage route should avoid intervening blood vessels, and transpleural drainage is a (relative) contraindication. Transsplenic drainage should also be avoided. The drainage of superficial parenchymal abscesses has a higher complication rate. In planning the access route, it is wise to maintain a safety margin of approximately 5 to 10 mm from vessels, bowel, ureters, and nerves.37 Particular attention should be given to the stage of the abscess (from phlegmonous inflammation to the formation of an abscess membrane).
Combined Procedures, Multiple Abscesses
Given the current interdisciplinary trend, there will be an increasing reliance on combined percutaneous, endoscopic, endosonographic, and laparoscopic approaches in the future. For the present, various endosonographic techniques make it possible to perform ultrasound-guided biopsies transvaginally and through the upper and lower gastrointestinal tract.38,39 These routes are described in Chapter ▶ 22 and are not detailed here. Whenever possible, retroperitoneal lesions should be approached through a posterior route outside the abdominal cavity.
When multiple abscesses have formed in one organ, their communication should be assessed before the procedure. With multiple liver abscesses, for example, it may be necessary to place drains at several sites or use a combination of percutaneous and transpapillary approaches. Moreover, it is important to consider not only the extent of abscess formation but also the sensitivity of the target organ.
Compartment Syndrome
Peritonitis and the less common abdominal compartment syndrome after a spontaneous perforation (or more commonly after endoscopy) may create a life-threatening situation that requires a swift response. Percutaneous drainage can be established at any number of sites in the abdominal cavity, and the route with the lowest risk should be chosen.40 This decision is aided by preliminary ultrasound to exclude possible collateral vessels in the abdominal wall. The abdominal cavity can be decompressed through an ordinary large-bore IV catheter. When dealing with a superficial abscess (e.g., abdominal wall abscess), ultrasound can direct the placement of soft drainage catheters without tissue injury.41
Suture Fixation
Once a catheter has been placed, it can be secured at skin level with a retention plate or suture. We prefer the latter method, in which a U-shaped stitch is passed through the skin approximately 5 to 10 mm from the catheter entry site and the suture material is fixed directly to the skin with three surgical knots. Another knot is tied approximately 10 mm above that point, whereupon the suture is looped around the drainage catheter and tied down, creating a total of three knots (▶ Fig. 15.11).
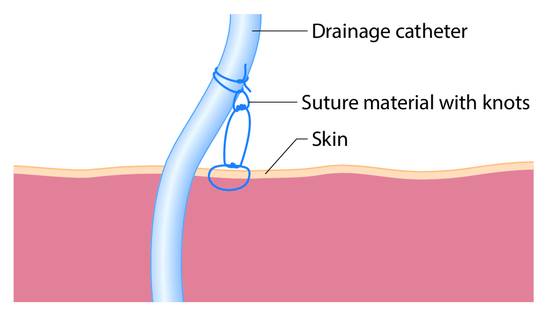
Fig. 15.11 Placement of the suture knots for catheter fixation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree