Thoracic Interventions
The chest wall, including the thoracic inlet and axillae, can be defined in exquisite detail with small, lightweight, high-resolution ultrasound transducers and modern ultrasound technology (▶ Fig. 24.1).
Pulmonary lesions can be visualized if they are in contact with the visceral pleura or are accessible to ultrasound scans through a sound-conducting medium (pleural effusion, atelectasis).1–4
Approximately 75% of clinically significant mediastinal masses in adults are located in the anterior and middle mediastinum and can be imaged sonographically from a suprasternal or parasternal site (left and right lateral decubitus).5
Only a few thoracic masses can be accurately classified etiologically on the basis of typical sonographic findings. Often a definitive diagnosis will require additional biochemical, microbiologic, cytologic, or histologic evaluation. The specimens necessary for these studies can be acquired by various means:
Percutaneous aspiration or core biopsy (ultrasound- or CT-guided)
Endoluminal access (bronchoscopy, endoluminal ultrasound)
Surgical access (mediastinoscopy, mediastinoscopic ultrasound, thoracoscopy, or open exposure)
24.1 Advantages of Ultrasound-Guided Interventions
Masses that are detectable by ultrasound are usually accessible to percutaneous biopsy unless the targeted lesion or needle path cannot be clearly visualized.
In the chest as elsewhere, an ultrasound-guided intervention offers definite advantages over CT guidance in suitably selected cases6,7:
The procedure can be performed quickly and at bedside.
There is no radiation exposure to the patient, physician, or staff.
The needle can be introduced in any direction and its path can be observed continuously.
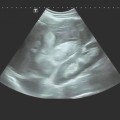
Ultrasound guidance can protect blood vessels (color Doppler), nerves (nerve plexuses at the thoracic inlet, ▶ Fig. 24.1), and aerated lung from injury (low incidence of pneumothorax) (▶ Fig. 24.2).
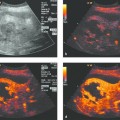
Active tumor elements (color Doppler, contrast agents) can be selectively targeted and sampled with a high success rate (▶ Fig. 24.3).
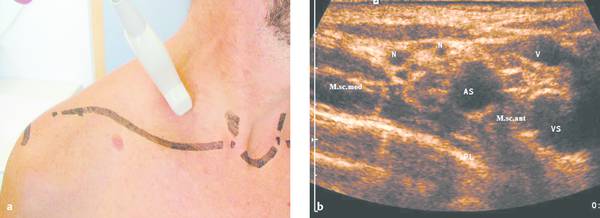
Fig. 24.1
a The thoracic inlet is examined sonographically in sagittal, axial, coronal, and lesion-adapted planes. Small high-resolution ultrasound probes, preferably with a sector image format (on a system with variable electronic formatting), are advantageous for displaying the complex anatomy about the thoracic inlet. The scan plane shown in the figure can display the brachial plexus and subclavian artery in the anterior scalene interval.
b Ultrasound image of the region scanned in panel a. The structures between the middle scalene muscle (M.s.c. med.) and anterior scalene muscle (M.s.c. ant.) are the subclavian artery (AS) anteriorly and a cross section of the hypoechoic nerve fascicles of the brachial plexus (N) posteriorly. It is important to preserve these neurovascular structures during the percutaneous biopsy of lymph nodes, apical lung tumors, etc., calling for a skilled biopsy technique. VS, subclavian vein; PL, summation echo from the parietal pleura, visceral pleura, and lung surface.
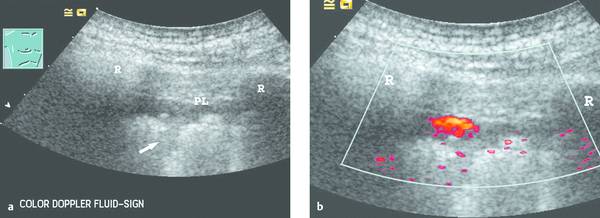
Fig. 24.2
a The parietal pleura (PL) appears as a slightly echogenic line. Subpleural deposits are visible along the irregular lung surface (arrow). A hypoechoic band appears between the lung surface and parietal pleura. Even the moving image (deep respiratory excursions) could not positively distinguish between a small effusion or a solid mass. R, rib.
b Power Doppler image during a deep respiratory excursion demonstrates the typical color Doppler fluid sign. This “motion artifact” is caused by moving fluid (low pulse repetition frequency, sensitive machine setting). Even tiny pleural effusions can be detected, and lesions can be precisely targeted to avoid unproductive biopsies.
Peripheral lung tumors can be distinguished from pneumonic or atelectatic lung areas by color Doppler and contrast-enhanced ultrasound (less production of motion artifacts).8,9
24.2 Indications
Although any sonographically visible mass can be biopsied in principle, an ultrasound-guided percutaneous biopsy is indicated only if it will have therapeutic implications (e.g., chemotherapy or radiation) or is expected to yield important prognostic information. When a peripheral tumor suspicious for malignancy is detected in an operable patient, it should not be biopsied but should undergo primary resection. There is no point in confirming an already established or plausible diagnosis. Biopsy should also be withheld if the same information can be obtained by less invasive means.10–13
The following indications are recognized for percutaneous biopsy or drainage procedures in the chest:
The investigation of fluid collections in the pleural space (benign/malignant effusion, empyema). Very small effusions can be distinguished from solid pleural thickening at once by the “fluid color sign” on color Doppler imaging (▶ Fig. 24.2); this avoids unproductive needle aspirations.9 Individual chambers in loculated pleural effusions can be selectively aspirated if empyema is suspected.
Paracentesis and pleural drainage for malignant and bloody pleural effusions. Early diagnostic confirmation and treatment of pleural empyema can prevent significant formation of septa. If septa have already formed, they can be lysed by the instillation of urokinase.3
Chest wall masses (tumors, hematomas, abscesses) including masses and destructive lesions of the ribs and sternum
Pleural masses
Peripheral lung changes in contact with the chest wall (lung tumor, pneumonia, lung abscess)
Mediastinal lesions, especially when located in the anterior or superior mediastinum
24.3 Contraindications
A severe coagulation disorder with INR >2 and platelet count <50 × 109/L is an absolute contraindication to a thoracic intervention.
Relative contraindications: bullous pulmonary emphysema and pulmonary hypertension. Severe respiratory compromise and poor blood gas values would contraindicate percutaneous drainage unless the intervention can improve the patient’s status.14
Percutaneous biopsy or drainage should be withheld if the intended target cannot be visualized or a safe needle path cannot be confirmed.15
24.4 Selection of Materials
24.4.1 Ultrasound Technology
High-frequency transducers (7.5 to 12 MHz) have excellent near-field resolution for detailed visualization of the chest wall. A small transducer footprint (4–6 cm) facilitates acoustic coupling and the biopsy procedure itself. Trapezoid imaging with an expandable field of view improves visualization of the intercostal space and of pleural areas bordering the ribs. Sector-type probes with a small footprint are advantageous for scanning pulmonary lesions.
Active tumor sites can be detected more sensitively by color Doppler and contrast-enhanced ultrasound, enabling the sites to be biopsied with greater precision and a higher success rate (▶ Fig. 24.3).16 These techniques can also be used to assess the efficacy of treatment, such as the palliative radiofrequency thermal ablation (RFTA) of chest wall tumors (e.g., metastatic hepatocellular carcinoma [HCC]).
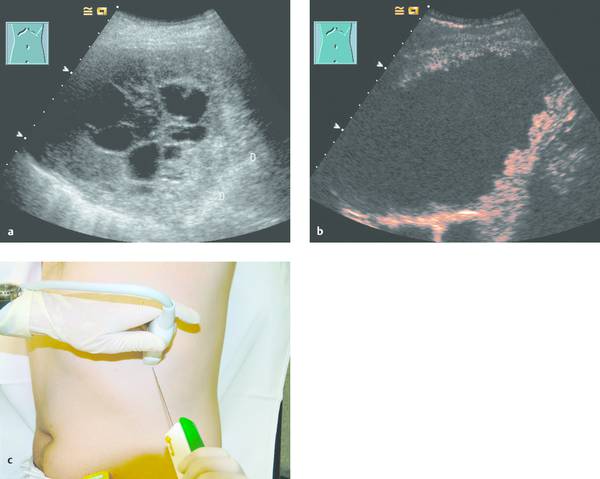
Fig. 24.3
a Where radiographs showed opacity in the left lower lung zone, B-mode ultrasound shows a solid-appearing mass with central cystic components. Color Doppler showed no definite blood flow even at a very sensitive setting. D, diaphragm.
b Contrast-enhanced ultrasound with a low mechanical index (low MI) shows enhancement confined to the peripheral portions of the mass.
c The areas that enhanced (with SonoVue [Bracco]) were selectively biopsied with a BioPince needle (Peter Pflugbeil). Histology revealed adenocarcinoma.
Color Doppler ultrasound can identify the mammary arteries and other blood vessels that must be spared in percutaneous needle procedures.5
24.4.2 Biopsy Devices
Economically priced needles without a stylet are satisfactory for aspiration cytology. No. 1 needles with an outer diameter of 0.9 mm (20-gauge) like those used for drawing blood have good echogenicity and produce a high-amplitude double echo at the needle tip. These needles can also be used for the aspiration of fluid collections (Sterican, Braun Melsungen).
Cutting biopsy needles can harvest tissue cores for histologic examination.
Coordinated manipulations of the ultrasound probe and needle tip are most easily accomplished with a “one person–one hand” technique. It is easier to maneuver the instruments in three dimensions, and needle tip adjustments can be made more quickly.7
Automated one-hand needle systems, or biopsy guns, are particularly suitable for thoracic lesions. They facilitate targeting, shorten the procedure time, and the fast needle stroke tends to penetrate rather than deflect the lung tissue. This leads to better biopsy results and lower complication rates, including a lower incidence of pneumothorax.
Several biopsy needles, all substantially equivalent, are available on the market, but it is best for an operator to become familiar with one needle type.
Currently we prefer the BioPince principle (BioPince Full Core Biopsy Instrument, Peter Pflugbeil) in which a special retention clip traps the tissue core inside the needle. Larger tissue cores can be harvested with this needle and retrieved safely.
A needle diameter of 1.2 mm provides a significantly better yield for histologic evaluation than a fine needle diameter (<1 mm), with only a minimal increase in complication rate.
The Trucut principle captures the tissue core in a specimen notch so that it cannot be lost (Trucut Needle, Bard). Disadvantages are that the tissue cores are short and thin relative to the needle diameter, and the system is relatively difficult to handle.
The Autovac single-use biopsy system (Surecut principle) by Bard (formerly Angiomed) is more favorably priced than the BioPince needle. The tissue core is excised by a rapid forward thrust of the needle. When the needle is withdrawn, the tissue core is retained in the needle by suction. In contrast to the BioPince needle, the specimen may be lost if the suction mechanism fails (with risk of tumor seeding in the needle track!) (▶ Fig. 24.4).
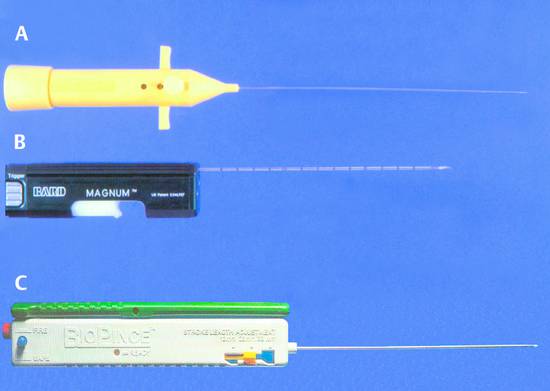
Fig. 24.4 This photograph illustrates three types of biopsy guns. The desired biopsy depth (measured sonographically) can be set on each device.
a Autovac single-use biopsy system (Bard, formerly Angiomed). The tissue core is excised by a rapid forward movement of the needle and is retained by suction.
b Reusable biopsy gun with Trucut needle inserted (Bard). The Trucut needle captures the tissue sample in a specimen notch so that it cannot be lost when the needle is withdrawn. This device yields only a half-diameter core, however, and is somewhat cumbersome to use.
c BioPince biopsy gun (Pflugbeil-Amedic). A retention clip traps the full core inside the needle so that it cannot be lost.
Source: Reprinted from Chest Sonography, 3rd ed. (ed. Gebhard Mathis), 2011 with kind permission of Springer Science and Business Media.
It is rarely necessary to use needle diameters larger than 1.2 mm. Simple aspiration needles are used for highly viscous fluids. A large-volume Trucut needle can be used for histologic differentiation, especially of benign chest wall lesions and occasionally of interstitial lung changes.
The diameter of a drainage catheter depends on the viscosity of the fluid collection. In principle, the drain can be placed by the trocar or Seldinger technique. The trocar technique is most commonly used in the chest (▶ Fig. 24.5).
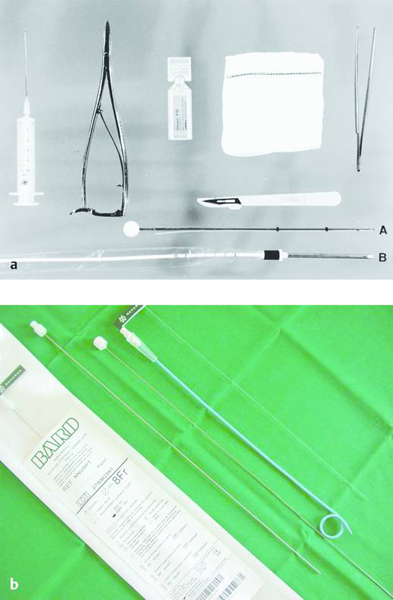
Fig. 24.5
a Pleural drainage set (trocar technique). A: classic trocar catheter (Argyle); small- and large-bore catheters are available. B: small-bore Pneumocat catheter (12F, Intra).
b Navarre universal drainage catheter (Bard-Angiomed, 8–12F). This is our favorite catheter at present. It has many advantages: it can be introduced directly through a small stab incision; it is easily inserted without prior dilatation; it is kink-resistant; it rarely clogs; and it has pigtail retention.
Source: Reprinted from Chest Sonography, 3rd ed. (ed. Gebhard Mathis), 2011 with kind permission of Springer Science and Business Media.
Available Devices
Sterican single-use injection needle, No. 1 (G 20/0.9 × 40/80 mm). B. Braun Melsungen AG, D-34209 Melsungen, Germany.
Sonocan single-use set for ultrasound-guided full-core biopsy (G 20/0.9 × 100/150 mm), B. Braun Melsungen. Supplier: Nicolai GmbH & Co. Ostpassage 7, D-30853 Langenhagen, Germany.
BioPince single-use full-core biopsy gun (G 18/1.2 × 100/150 mm), InterV-MDTech, Gainesville, Florida, USA. Supplier: Pflugbeil-Amedic.
Max-Core disposable core biopsy instrument (G 20–16/0.9–1.2 × 100/160 mm). Bard GmbH, Wachhausstrasse 6, D-76227 Karlsruhe, Germany.
Magnum reusable core biopsy instrument, Bard.
Magnum disposable needles (G 20–16/0.9–1.2 × 100/160 mm), Bard.
Navarre Universal Drainage Catheter with nitinol (6–12 F × 30 cm), Bard. Universal adapter with Luer lock, Bard.
Argyle trocar catheter (12F–17F/4–6 mm). Sherwood Medical, Tullamore, Ireland. EC rep: Gosport, PO 13 OAS, UK.
Argyle Sentinel Seal Chest Drainage Unit. Tyco Healthcare, Tullamore, Ireland.
24.5 Preparations
The basic equipment for percutaneous biopsy or drainage procedures includes syringes, cannulas, biopsy needles, gloves, local anesthesia, and sterile draping material. As in any intervention, the patient must be duly informed about the steps in the procedure and its risks.
Coagulation status should be assessed (except for chest wall biopsies). Antiplatelet drugs are discontinued 4 to 5 days before an elective procedure. For urgent procedures (e.g., abscess, symptomatic pleural effusion), the risk should be determined on a case-by-case basis.
The initial diagnostic work-up consists of thoracic ultrasonography and may include a chest radiograph or CT examination. Next, four determinations are made: the intended target, the puncture site, the direction of needle insertion, and the needle path.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree