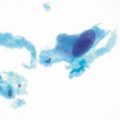
Fig. 8.1
Histopathology revealing gastritis with foveolar hyperplasia, vascular ectasia, granulation tissue, and scattered yttrium 90 selective internal radiation microspheres.
Risk Factors
Some patients have increased susceptibility to radiation enteropathy. However, late bowel injury is generally multifactorial. It is uncommon to have a small bowel injury in preoperative rectal or cervical cancer patients, but very common in postoperative patients. Given that there are a number of risk factors (medical comorbidities and anatomical/surgical comorbidities), any injury after multimodality cancer therapy should be considered “treatment related,” and not solely based on radiation toxicity itself [18]. An example of this is seen in patients with small bowel toxicity after receiving 5-fluorouracil (5-FU) and pelvic radiotherapy. Although 5-FU is known to cause GI comorbidity [19], it is common to describe chemoradiation-induced small bowel toxicity as radiation enteropathy. One can argue, however, that the correct term should be “treatment-related enteropathy.”
Besides medication, medical comorbidities may also increase the risk for radiation enteropathy and these include diabetes, hypertension, and cardiovascular disease. The risk is higher in these patients due to preexisting vascular damage or occlusion [20]. Radiation damage accelerates underlying vascular disease by increasing the severity of endarteritis and ultimately, tissue fibrosis. Patients with collagen vascular disease and inflammatory bowel disease also have a higher risk of acute as well as chronic radiation-induced injury. These conditions and their associated small intestinal pathology result in poorer GI tolerance to RT [21–23].
Anatomical variation also increases a patient’s risk for treatment-related enteropathy. Women, older patients, and thin patients may have augmented volumes of the small intestine in the pelvic cul-de-sac, increasing the probability of radiation injury following treatment for pelvic malignancies [24]. Furthermore, patients with a history of pelvic inflammatory disease or endometriosis also appear to be at a higher risk of small intestinal injury [25, 26]. In addition, patients with a history of previous intra-abdominal surgery or peritonitis are especially susceptible, as immobility and restriction may cause areas of the small intestine affected by adhesions to be consistently exposed to higher radiation fractions [27, 28].
Effects of Radiation Dosage
The volume of radiation given and other risk factors, most importantly surgery, total dose, treatment time, and technique of pelvic irradiation all influence the likelihood of occurrence of small intestinal damage. Patients can generally receive 45–50 Gy in 1.8–2 Gy daily fractions to a pelvic field without significant bowel toxicity [29]. For postoperative patients, radiation of 45–50 Gy over 5 weeks is associated with an approximate 5 % incidence of small bowel obstruction requiring surgery, while at doses greater than 50 Gy, the incidence of toxicity rises to as high as 50 % [24]. When lesser volumes of small intestine are exposed to radiation, the degree of toxicity is diminished [30]. This concept was confirmed in a study of rectal cancer patients treated preoperatively with chemoradiotherapy showing a strong correlation between the occurrences of severe diarrhea and irradiated small bowel volume. In this study, 40 patients receiving concurrent 5-FU-based chemotherapy and pelvic irradiation for the treatment of rectal carcinoma had treatment—planning computerized tomography (CT) scans with small bowel contrast. A median isocentric dose of 50.4 Gy was delivered using a posterior–anterior and opposed lateral field arrangement. Bowel exclusion techniques were used including prone position on a vacuum bag cradle to allow anterior displacement of the abdominal contents and bladder distension to limit radiation exposure. The volume of small bowel receiving each dose between 5 and 40 Gy was recorded at 5-Gy intervals. Ten patients (25 %) experienced high-grade acute small bowel toxicity . A statistically significant association between the development of acute small bowel toxicity and the volume of small bowel irradiated was found at each dose level. The volume of small bowel receiving at least 15 Gy was strongly associated with the degree of toxicity. The authors advised that limiting the volume of small bowel receiving greater than 15 Gy may significantly improve treatment tolerance [31].
Furthermore, as noted previously, the combination of radiation and chemotherapy increases the risk of small intestinal toxicity as chemotherapy can cause significant small bowel toxicity without radiation. In a randomized trial delivering 40–48 Gy to 202 patients with rectal cancer, the incidence of severe small bowel complications were significantly higher in patients who received postoperative 5-FU and RT than in those who received radiation alone (35 % vs 15 %) [32, 33]. The infusion modality of chemotherapy with radiation has also been studied, revealing higher risk in those who receive bolus doses of chemotherapy instead of continuous infusion. In one study, 660 patients with TNM stage II rectal cancer received intermittent bolus injections or protracted venous infusions of 5-FU during postoperative radiation to the pelvis. With a median follow-up over 46 months, patients who received a protracted infusion of fluorouracil had significantly increased time to relapse and improved survival in comparison to bolus doses. Furthermore, although continuous 5-FU with radiation to 50.4 Gy in 1.8-Gy fractions was associated with an increase in acute diarrhea, there was no increased risk of chronic or severe small bowel toxicity when compared to bolus 5-FU therapy [34]. Although the addition of concurrent chemotherapy increases the acute toxicity of external radiotherapy, its contribution to late bowel toxicity is still poorly understood [8].
Diagnosis
The diagnosis of radiation enteropathy is a clinical diagnosis made by a history of prior radiation exposure, suggestive clinical features, and supportive radiologic findings. Details of the patients’ previous radiation history (including last dose, fractioned and total doses, and distribution of the radiation field) should be obtained during the initial evaluation.
Diagnosing radiation enteropathy may be difficult as it is not simply a function of the radiation dose from previous treatment and involves a number of presumed physiologic and functional changes in the small bowel based on the patient’s medical risk factors. One study showed that radiation enteropathy is not a single disease. In this study, a total of 265 patients were referred with urologic, gynecologic, and GI cancers and after therapy it was found that one-third of the patients’ symptoms were not related to RT itself [35]. More than half of the patients had at least two diagnoses, while the remaining were determined to be considered to be due to radiation damage solely. As a result, prior to beginning a work up to determine if radiation enteropathy is the direct result of intestinal damage or a codependent factor, other disease states need to be ruled out or as potential contributors. Due to limited treatment options that directly improve radiation enteropathy, emphasis on finding additional factors that may be accentuating symptoms is advised. These include Infectious, inflammatory, or medication-related injury, which can be treated with additional medication or the discontinuation of offending agents.
Upper GI radiography with barium and CT have been the mainstay tests for diagnosing treatment/radiation-related enteropathy. Upper GI series with small bowel follow-through can be used as an initial test to evaluate the extent of disease, but is not as sensitive as enteroclysis [36]. Enteroclysis, involving infusion of contrast material via nasoenteric tube into the small bowel using a pump, provides more complete visualization of the small bowel mucosa in comparison to standard upper GI series [37]. However, its sensitivity and specificity have not been well defined in the setting of suspected radiation enteropathy.
Abdominal CT is commonly used for the evaluation of small intestinal radiation injury, particularly in evaluating areas of suspected stricture. CT enteroclysis produces superior imaging compared to conventional CT, with a sensitivity and specificity of 88 and 82 %, respectively, when evaluating low-grade and intermittent obstruction [38, 39].
Radiographic abnormalities detected on small bowel series and CT typically include thickening of the small bowel folds and the intestinal wall with or without intramural hemorrhage, edema, and mucosal ulceration (Figs. 8.2, 8.3, 8.4). In chronic radiation injury, stenosis, adhesions, or fistulas can be seen. In some cases, features classically labeled as “ribbon” or “toothpaste” bowel are displayed, reflecting the small intestinal mucosal destruction.
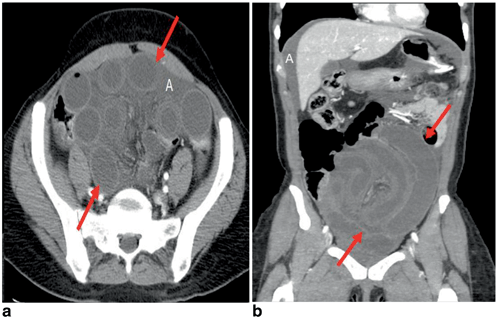
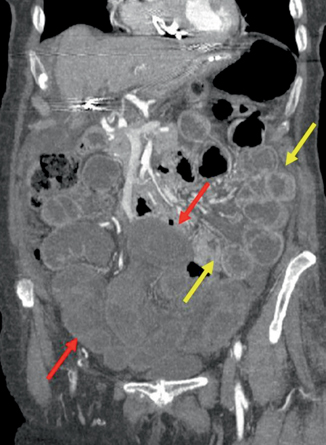
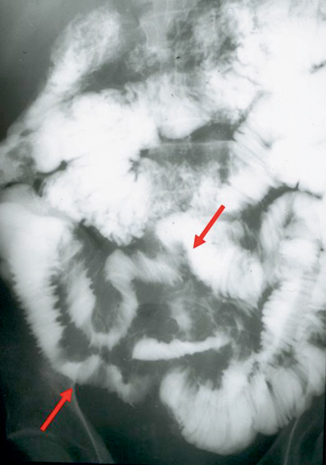

Fig. 8.2
Diffuse acute radiation enteritis. Axial (a) and coronal (b) multiplanar reformatted CT images show diffuse mural thickening of the small bowel with submucosal edema and increased intraluminal secretions with ascites. (Courtesy of Richard Gore, MD)

Fig. 8.3
Chronic radiation enteritis—poor perfusion and mural thickening on this coronal reformatted CT image (red arrows). Notice the bowel not within the mantle enhances normally (yellow arrows). (Courtesy of Richard Gore, MD)

Fig. 8.4
Chronic radiation enteritis—small bowel follow through shows mucosal irregularity, thickening of the valvulae conniventes, and minimal separation of distal ileal segments (red arrows). (Courtesy of Richard Gore, MD)
Magnetic resonance (MR) enterography for the evaluation of Crohn’s disease is well described in literature, and has an evolving role in the evaluation of other small-bowel diseases including radiation enteritis. MR enterography may be particularly useful for the evaluation of intermittent and low-grade small-bowel obstructions in patients with radiation enteropathy. Advantages of MR over CT as an imaging modality include superb contrast resolution, lack of exposure to ionizing radiation, and the use of several intravenous contrast media with improved safety profiles. Limitations of MR include higher cost, less availability, variability of image quality, and lower spatial resolutions (the measure of how closely lines can be resolved in an image). Comparisons between MR enterography and MR enteroclysis are in process [40], although some studies suggest that MR enteroclysis results are comparable and possibly more sensitive than CT enteroclysis [37].
Capsule endoscopy can be used to diagnose radiation enteropathy, but is generally avoided given the risk of the capsule becoming trapped in a strictured segment requiring surgical removal. Colonoscopy with intubation of the ileum can be helpful if ileal involvement is suspected. Endoscopic features consistent with radiation injury include mucosal atrophy and edema, friability, pallor, and the presence of telangiectasias [41], Unfortunately, even if the ileal mucosa appears to be normal, patients can still be at risk for complications of radiation enteropathy including spontaneous perforation [42]. Mucosal biopsies are usually not diagnostic, but can eliminate other causes of small bowel injury including inflammatory bowel disease, infections, and nonsteroidal anti-inflammatory drug (NSAID)-induced damage.
Prevention
If RT is anticipated after surgery, attempts have to be made at the time of surgery to displace the bowel outside the radiation field [43]. One technique is the surgical placement of polyglycolic, biodegradable mesh that moves the intestines out of the pelvis [44, 45]. In two published studies, one with 8 children and another including 60 adults receiving RT, placement of a mesh reduced the occurrence of delayed symptoms of radiation enteritis. In both of these studies, radiological evaluation was used to confirm that the small bowel was out of the pelvis and the area of irradiation posttreatment. A reduction of 50 % of the volume of the small bowel exposed to the radiation has been demonstrated with placement of a mesh during surgery, allowing a higher dose of radiation to be given postoperatively where indicated [46]. Other techniques such as pelvic reconstruction, omentoplasty, and transposition of the colon may also significantly decrease the volume of bowel exposed to RT [46–49]. In many patients, treatment in the prone position with a special “belly board” (a customized polyurethane and styrofoam bowel immobilization mold), allows the displacement of the small intestines out of the radiation field (Fig. 8.5) [50, 51].

Fig. 8.5
Koilia Mikros Belly Board allows displacement of the small intestines from the radiation field during treatment. (With permission from CDR Systems)
Advances in computer technology have also led to the development of three-dimensional (3D) RT planning systems and computer-controlled RT delivery [52–54], which theoretically will improve the efficiency. With these techniques, external beam RT can be planned and delivered with a reduction in complications and cost. Intensity-modulated RT (IMRT) requires the same careful 3D radiation treatment planning, but takes it a step further by utilizing variable, computer-controlled intensities within each RT beam. As a result it can achieve a higher degree of accuracy in conformation of the radiation to the planned target, and spare normal tissue. One phase II trial, studying 83 patients with endometrial and cervical cancer receiving IMRT, showed lower hematologic toxicity by reducing the volume of bone marrow irradiated [55]. Another retrospective review of patients with rectal cancer comparing 61 patients treated with conventional RT versus 31 patients with IMRT showed a significant reduction in acute lower GI toxicity [56]. Yet another study of patients with prostate cancer receiving pelvic radiotherapy found that patients receiving IMRT had a 40 % relative reduction in the volume of bowel receiving 45 Gy in comparison to conventional two-dimensional (2D) planning. This newer technology has promise in reducing the rates of acute and late GI morbidity when using RT.
Preventive therapeutic strategies currently being studied include investigation of antioxidants, free-radical scavengers, cytokine modification, enterotrophic strategies, novel anti-inflammatory agents, modulators of intraluminal contents, modulators of endothelial dysfunction, as well neuroimmunomodulators [57].
For further details, please see Chap. 4-Prevention of Injury from Pelvic Irradiation.
Treatment
Treatment for acute and chronic radiation enteropathy has been directed to relieve symptoms and for management of disease complications. Unfortunately, specific medical treatment has been driven by small clinical trials, retrospective studies, and case series. At present, no pharmacotherapy has been shown to alter the natural history of the condition.
A variety of symptomatic treatments for radiation enteropathy have been suggested (see Table 8.1). Loperamide has been used to control the symptoms of acute and chronic disease. In one study of patients, loperamide 3 mg twice a day for 14 days was associated with a significant reduction in the frequency of bowel movements, slower intestinal transit, and improvement in the absorption of bile acids [58]. A study of oral sucralfate in patients receiving pelvic irradiation showed a decrease in frequency and improvement in consistency of bowl movements. The study was a double-blind, placebo-controlled trial including 70 patients with prostate or bladder cancer without distant metastasis. Radiotherapy was conventionally delivered. Dose granules of sucralfate or placebo were dispensed to each patient 2 weeks after radiation and continued for 6 weeks. The frequency of defecation and stool consistency was significantly improved by sucralfate, where 14 patients in the placebo group versus 3 patients in the sucralfate group ultimately required loperamide. Furthermore, in this study, not only did loperamide reduce acute symptoms occurring during radiotherapy, but was also effective for chronic symptoms 1 year after completion of RT [59].
Table 8.1
Therapy for radiation enteropathy
Therapy | Dose | Indication |
|---|---|---|
Loperamide | 4 mg twice a day (titrate as needed) | Diarrhea |
Sucralfate | 1 g four times a day (titrate as needed) | Diarrhea |
Cholestyramine | 2–4 g twice to four times a day (titrate as needed) | Diarrhea (especially with bile salt diarrhea) |
Rifaximin | 1200 mg a day for 7–10 days | Small intestinal bacterial overgrowth |
Amoxicillin/clavulanate | 30 mg/kg a day for 7–10 days | Small intestinal bacterial overgrowth |
Metronidazole with Cephalexin or Trimethoprim-sulfamethoxazide or Gentamycin | Metronidazole (20 mg/kg/day) Cephalexin (30 mg/kg/day Trimethoprim-sulfamethoxazide (10–12 mg/kg/day) Gentamycin (10 mg/kg/day) *For 7 to 10 days | Small intestinal bacterial overgrowth |
Norfloxacin
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|