Acute and Inflammatory Disorders of the Lower Intestinal Tract
Ramesh S. Iyer, MD
LEARNING OBJECTIVES
1. Identify necrotizing enterocolitis on abdominal radiographs.
2. Describe US and CT features of appendicitis.
3. Recognize intussusception on US.
4. Discuss a general approach to enema reduction for treatment of intussusception.
5. Identify Meckel diverticulum on a pertechnetate scan.
6. List at least two differences between Crohn disease and ulcerative colitis.
7. Describe typical imaging features of inflammatory bowel disease on CT or MR enterography.
INTRODUCTION
The disorders included in this chapter account for a large portion of pediatric imaging in acute care settings. Though etiologies are varied, these conditions may all result in acute bowel obstruction or inflammation and may need urgent intervention. Radiologists should be familiar with their respective imaging features and diagnostic workup to facilitate prompt appropriate therapy.
NECROTIZING ENTEROCOLITIS
Necrotizing enterocolitis (NEC) predominantly afflicts preterm neonates in the intensive care unit (ICU) setting. It is by far the most common cause of acquired gastrointestinal morbidity and mortality in these patients.1,2 Ninety percent of NEC occurs in neonates who are preterm (less than 34 weeks of gestational age) with birth weights less than 1,500 g.3,4 NEC typically occurs between 1 and 3 weeks of age.5 Age of onset for NEC is inversely related to gestational age and weight at birth. That is to say, smaller and more preterm infants present with NEC later in their first month of life.1, 2, 3, 4 and 5 Ten percent of NEC occurs in full-term infants, with disease onset during the first few days of life.5 The pathophysiology for NEC has not been fully elucidated yet. It is believed that an ischemic insult damages intestinal mucosa, causing bacterial wall invasion. Subsequently, an inappropriately strong inflammatory response results in bowel necrosis.2,6,7 NEC in term infants is virtually always a complication for patients already admitted to the ICU for another reason.8
Abdominal distension and bloody stools in a preterm neonate is often the relevant clinical scenario learned by radiologists. However, perinatologists track additional clinical indicators and categorize signs and symptoms of NEC using the modified Bell criteria. Stage 1 criteria (suspected NEC) include increased gastric residuals, lethargy, bradycardia, and temperature instability, among other nonspecific signs. Stage 2 (definite NEC) signs include grossly bloody stools and marked abdominal distension. In stage 3 NEC (advanced), the patient becomes progressively unstable with deterioration of vital signs and signs of septic shock.9
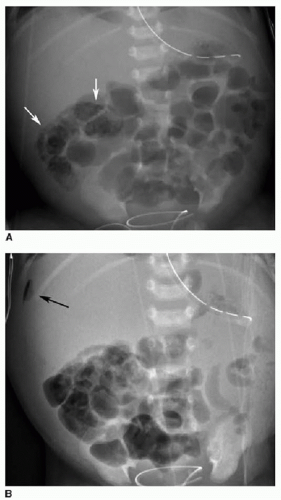
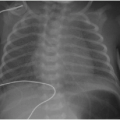
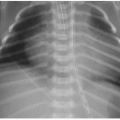
Abdominal radiography is the mainstay of imaging NEC. Serial radiographs are performed at 6- to 12-hour intervals for babies suspected of having NEC. Cross-table lateral or left lateral decubitus views should be included to look for pneumoperitoneum. Early or mild disease will manifest as either a normal bowel gas pattern or an ileus pattern with mild focal or diffuse gaseous distension. Persistent bowel distension, either generalized or focal, is the most common radiographic appearance of NEC (Figs. 15.1 and 15.2). The degree of distension typically correlates well with disease severity. Diffuse bowel distension may progress to focal or asymmetric distension of one or more loops. A fixed pattern of bowel gas over time is highly concerning for bowel ischemia. Pneumatosis is another definitive radiographic indicator of NEC (Figs. 15.1, 15.2 and 15.3). This most frequently occurs in the right lower abdomen but may occur anywhere within the intestinal tract. The bubbly lucencies of pneumatosis may be mistaken for those of stool and vice versa. Pneumatosis should be suspected when tiny lucencies are organized in a curvilinear pattern, following the expected arrangement of the bowel wall. These areas should be closely inspected on subsequent radiographs if there is trouble distinguishing between them from stool. Portal venous gas will appear as branching linear lucencies in the liver and can be a transient phenomenon with NEC (Fig. 15.2). Frank pneumoperitoneum is the hallmark of advanced disease (Fig. 15.3B).2,3,5, 6 and 7,9
Abdominal ultrasound (US) may be a useful adjunct modality in cases of NEC. The affected bowel wall is thickened and hypoechoic from edema. Color Doppler interrogation may yield either increased (inflamed) or decreased (ischemic) wall perfusion.
Intramural gas will appear as echogenic foci within the bowel wall with posterior acoustic shadowing. US will also identify ascites or focal fluid collections.2,3,5, 6 and 7
Intramural gas will appear as echogenic foci within the bowel wall with posterior acoustic shadowing. US will also identify ascites or focal fluid collections.2,3,5, 6 and 7
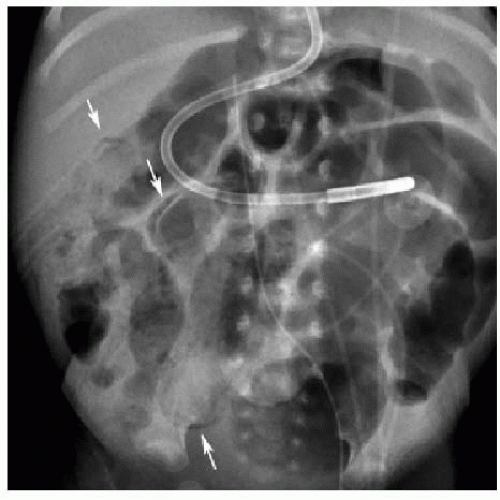 FIG. 15.1 • Necrotizing enterocolitis in a 3-week-old male. AP abdominal radiograph demonstrates pneumatosis in the right abdomen (arrows), and mild gaseous distension of featureless bowel loops. |
Medical management for NEC includes discontinuation of enteral feeds, nasogastric tube placement for decompression, fluid resuscitation, and intravenous antibiotics. Surgery is considered for patients who fail to respond to medical management or who have frank bowel perforation. Segmental resection of the necrotic bowel is performed. Complications of NEC include bowel strictures and short gut syndrome (Fig. 15.4).2,3,9
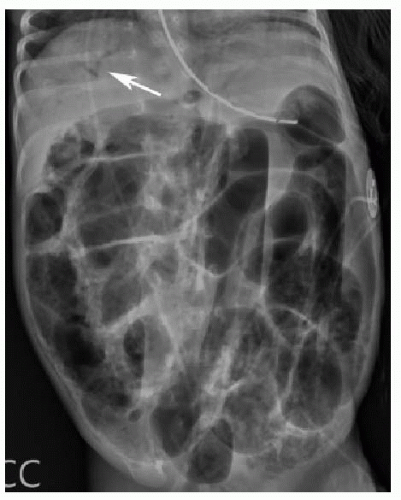 FIG. 15.2 • Necrotizing enterocolitis in a 2-week-old male. Left lateral decubitus abdominal x-ray showing widespread pneumatosis, diffuse bowel distension, and portal venous gas (arrow). |
APPENDICITIS
Acute appendicitis is the most common reason for abdominal surgery in children, as in adults. There are 70,000 to 90,000 cases of pediatric appendicitis annually in the United States. Incidence increases with age and peaks in adolescence.10 It is rare under 2 years of age.11 The process begins with luminal obstruction, usually by a fecalith. As intraluminal pressure increases, there is progressive venous outflow obstruction and wall ischemia in the appendix. In later or advanced cases, there is bacterial wall invasion, leading to gangrene and perforation.
The classic history for appendicitis is nausea, emesis, and crampy periumbilical pain that migrates to the right lower
abdomen, with tenderness at McBurney point. Unfortunately, the workup for appendicitis in children is often not this straightforward. Twenty to thirty percent of pediatric appendicitis cases feature atypical clinical presentations.11,12 Fever may be absent or very high, emesis and abdominal pain may be variably present, and variations in appendiceal position may alter pain patterns. Finally, young children may be less able to fully describe their symptoms. Thus, there is increased reliance upon imaging to achieve a diagnosis of appendicitis in children.
abdomen, with tenderness at McBurney point. Unfortunately, the workup for appendicitis in children is often not this straightforward. Twenty to thirty percent of pediatric appendicitis cases feature atypical clinical presentations.11,12 Fever may be absent or very high, emesis and abdominal pain may be variably present, and variations in appendiceal position may alter pain patterns. Finally, young children may be less able to fully describe their symptoms. Thus, there is increased reliance upon imaging to achieve a diagnosis of appendicitis in children.
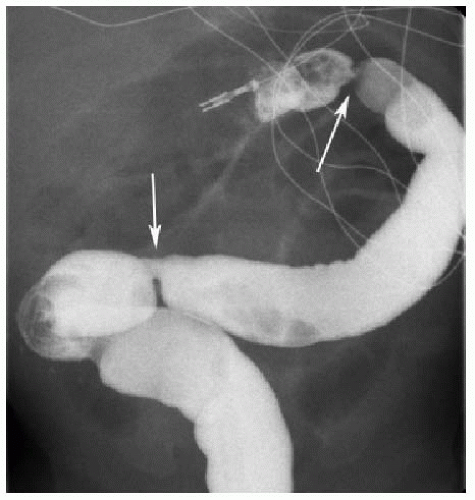 FIG. 15.4 • Post-NEC strictures in a 2-month-old female. Contrast enema shows two short-segment colonic strictures (arrows). The patient had NEC at 1 week of age. |
The imaging workup of appendicitis remains controversial. National and regional differences, as well as variation among practices in a single region, are barriers toward a single standard approach.13,14 The debate usually boils down to US or CT for initial evaluation. US benefits from less cost and the lack of ionizing radiation but is limited by operator dependence, which is a major barrier in certain practices.15 CT features slightly higher sensitivity and specificity but involves radiation and the risk of a contrast reaction.16 Several authors have advocated the use of a hybrid or tiered approach incorporating both modalities. In such a system, US is used initially, and CT is reserved for equivocal or technically challenging cases or when perforation is suspected.17, 18 and 19
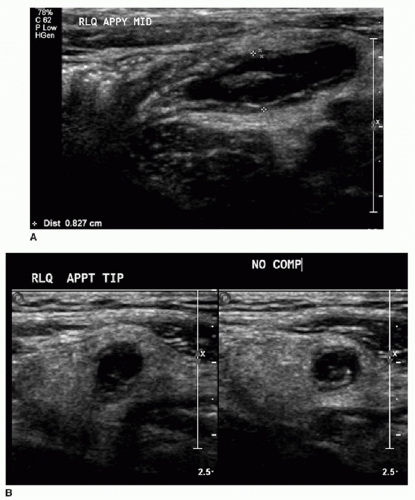
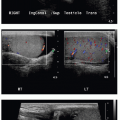
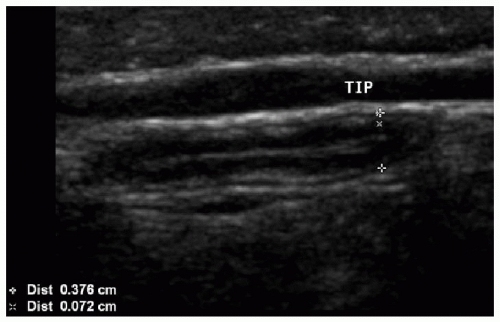 FIG. 15.5 • Normal appendix on US. Thin-walled tubular structure measures less than 6 mm in caliber, with a normal stratified mural architecture, and no surrounding inflammation. |
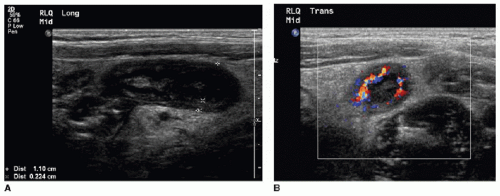
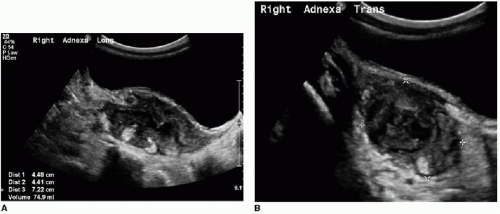
US should be performed with a high-frequency transducer (9 to 15 MHz) using a graded compression technique. A mean outer diameter (MOD) of 6 mm is the widely reported sonographic threshold used to distinguish a normal from an abnormal appendix. An appendix less than 6 mm in diameter is nearly always normal (Fig. 15.5).10,11,20 Some authors recommend using MOD greater than 7 mm to diagnose appendicitis, with reportedly high sensitivity and specificity.21 It is important to be aware that a greater than expected MOD can sometimes be misleading when normal appendices are simply distended by gas or fecal material. Therefore, more information than just the appendiceal diameter must be assessed to make an accurate diagnosis. Secondary signs of right lower quadrant inflammation are very helpful when assessing for appendicitis. This is particularly true when the appendix measures between 6 and 7 mm. Ancillary signs of appendicitis by US include increased echogenicity of the periappendiceal fat, hyperemia within or surrounding the appendix, lack of compressibility, loss of normal mural architecture, hypoechoic intraluminal contents, and the presence of an appendicolith (Figs. 15.6 and 15.7).
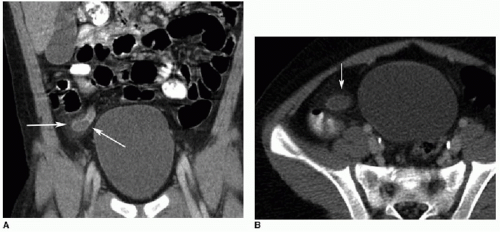
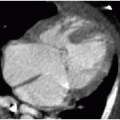
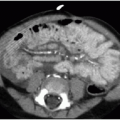
On CT, the normal appendix may vary in size between 2 and 11 mm. This is because the study is performed without compression and the appendix may distend with gas or stool.10 In general, with acute appendicitis on CT, the appendix exceeds 7 mm in diameter and has a thickened wall. There is lack of filling by luminal contrast (either oral or rectal) and surrounding inflammatory fat stranding (Fig. 15.8). Calcified fecalith and prominent surrounding mesenteric lymph nodes are variably present.11
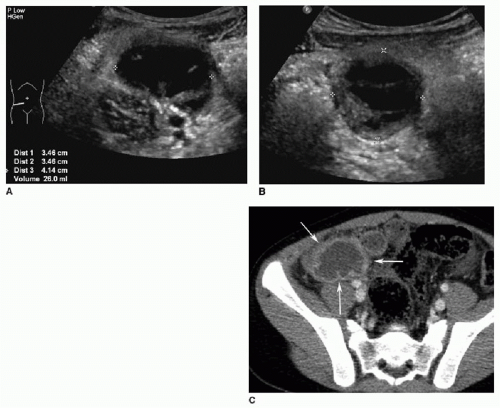
There are a few common themes across modalities when imaging appendicitis. First, the radiologist should make every effort to identify the entire appendix and specifically comment when there is incomplete visualization. This is particularly true with appendiceal US, which may be a challenging examination even for experienced sonologists. Second, it is important to identify any abdominal fluid collections suspicious for abscess (Fig. 15.9). Finally, in cases of perforated appendicitis, there is often distortion of normal anatomy, and the resultant inflammatory mass may mimic tumor or other pathology (Fig. 15.10).
Acute nonperforated appendicitis is managed surgically with an appendectomy. In cases of perforated appendicitis, IV antibiotics are administered and an interval appendectomy may be performed several weeks later.22 If there is a large periappendiceal abscess, image-guided percutaneous drainage is performed.
INTUSSUSCEPTION
Intussusception is the pathologic invagination or “telescoping” of bowel into itself. The intussusceptum is the invaginating or protruding segment of more proximal bowel, while the intussuscipiens is the receiving loop of the distal bowel. As the intussusceptum is propagated distally, the mesenteric vessels become compressed, leading to reduced bowel perfusion, venous congestion, and possibly necrosis.23 Intussusception usually occurs in children aged 3 months to 3 years, with a peak incidence between 5 and 9 months of age.24,25 Patients present with colicky abdominal pain, alternating lethargy and irritability, and emesis. Though infrequently present, the hallmark clinical sign of intussusception is “currant jelly” bloody stool representing sloughed mucosa. The classic clinical triad of colicky pain, currant jelly stool, and palpable abdominal mass is present in only 50% of cases of intussusception, with up to 20% of patients pain free at presentation.23,24 There is seasonal predilection for the condition, with higher incidence in spring and autumn. This supports the notion of a predisposing viral illness for intussusception.24
Approximately 90% of cases are “idiopathic” ileocolic intussusceptions. It is thought that lymphoid hyperplasia within the Peyer patches of the distal ileum, presumably in response to a viral infection, causes the vast majority of intussusceptions. Less common subtypes of intussusceptions involve only the colon (colocolonic) or small bowel (jejunojejunal or ileoileal). Approximately 5% of intussusceptions arise from a pathologic lead point. This should be suspected when the condition arises outside of the usual age range or in an atypical location. In young children, the most common pathologies in descending order are Meckel diverticulum, duplication cyst, and polyp. In older children, lymphoma is a likely lead point.26
Abdominal radiographs have overall limited sensitivity and specificity for intussusception.27 However, a left lateral decubitus view can improve the ability to diagnose or exclude the condition radiographically. If the cecum and ascending colon are well visualized and filled with either gas or stool, then there is a low likelihood of ileocolic intussusception (Fig. 15.11).28
A potential pitfall is gas within the small bowel or redundant sigmoid colon that can mimic a gas-filled cecum. Radiographic findings suggestive of intussusception include a soft tissue mass and/or absence of bowel gas within the right abdomen (Fig. 15.12). The “meniscus sign” is a crescent of colonic gas that outlines the intussusceptum and is highly suggestive of the diagnosis (Figs. 15.12 and 15.14).23,25,



A potential pitfall is gas within the small bowel or redundant sigmoid colon that can mimic a gas-filled cecum. Radiographic findings suggestive of intussusception include a soft tissue mass and/or absence of bowel gas within the right abdomen (Fig. 15.12). The “meniscus sign” is a crescent of colonic gas that outlines the intussusceptum and is highly suggestive of the diagnosis (Figs. 15.12 and 15.14).23,25,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree