Adult Hip
Paul Mallinson
Philip Robinson
INTRODUCTION
Advances in sonographic technology and increasing awareness of its capabilities have led to a rapidly expanding role for ultrasound in assessing the adult hip. Ultrasound provides a valuable addition or alternative to other imaging modalities due to its dynamic capabilities, rapid scan times, exquisite soft tissue detail (even in the presence of metal implants), and ability to guide interventional procedures. This chapter describes the anatomy, scanning technique, and pathologies of the adult hip and pelvis. Advantages and limitations of ultrasound when compared with magnetic resonance imaging (MRI) are also discussed.
ANATOMY AND TECHNIQUE
The optimal probe for scanning the hip depends on the depth of the structure being imaged and the size of the patient. High-frequency linear transducers of the order of 17 MHz provide excellent superficial soft tissue detail in smaller patients, but 12 MHz, 9 MHz linear, or even 2 to 5 MHz curvilinear probe may be required for deeper structures in larger patients. Start with the highest frequency and then reduce as required.
Anatomy and scanning techniques are best considered as four quadrants: anterior, posterior, medial, and lateral.
Anterior
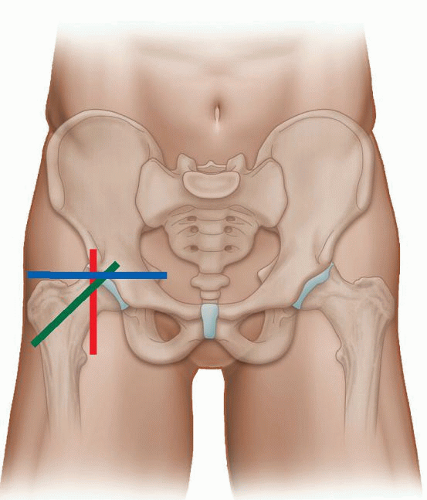
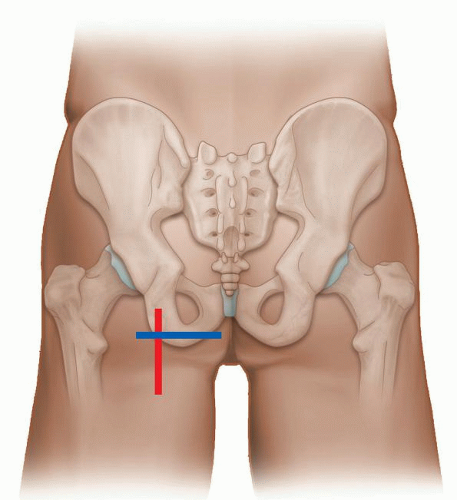
The anterior quadrant contains the anterior joint recess, iliopsoas tendon, rectus femoris, sartorius, and tensor fascia lata (TFL). (Examination of groin hernias is covered in the hernia section.) The examination is performed with the patient supine and transverse (TS) and longitudinal (LS) images are obtained (Fig. 6.1). The anterior joint recess is evaluated with the probe aligned obliquely along the femoral neck to look for the presence of an effusion or large anterior labral injury in the normally hyperechoic labrum. The hyperechoic iliofemoral ligament can sometimes be seen overlying the anterior capsule (Fig. 6.2A).
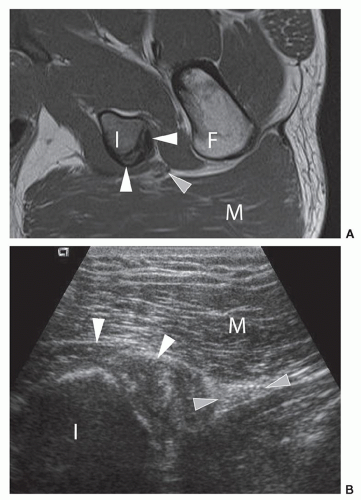
The iliopsoas muscle is scanned in true longitudinal and transverse views (Figs. 6.2 and 6.3). It lies superficial to the capsule and lateral to the femoral neurovascular bundle. The hyperechoic iliopsoas tendon lies on the deep and medial aspect of the muscle and inserts distally on the lesser trochanter. The iliopsoas bursa lies between the anterior joint capsule and tendon, but is not seen unless bursitis or fluid are present. Superficial to iliopsoas is sartorius, which can be traced distally from the anterior superior iliac spine (ASIS). Tensor fascia lata is superficial and lateral and also originates from the ASIS and iliac crest (Fig. 6.3). The TFL courses laterally to blend into the anterior aspect of the fascia lata. Superficial and medial to iliopsoas is rectus femoris, which originates from the anterior inferior iliac spine (AIIS). Longitudinal views of these muscles and tendons should be performed when evaluating for tendinopathy and muscle tears.
Posterior and Lateral
For anatomical purposes these two quadrants are considered together. They incorporate the gluteal muscles and tendons. The hamstring origins and sciatic nerve will also be considered here.
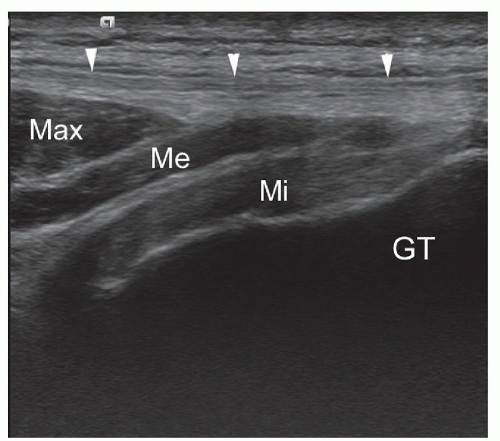
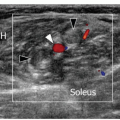
For gluteal assessment, the patient is initially scanned in the lateral decubitus position with a high-frequency probe. Gluteus medius (deep) and gluteus minimus (superficial) can be traced cranially down to the greater trochanter in both long-axis and short-axis scans (Fig. 6.4). The TFL is adjacent to the anterior margins of these two muscles, and gluteus maximus overlies the posterior portion of gluteus medius. The echogenic tendons of gluteus minimus and medius insert into the anterior and lateral aspects of the greater trochanter, respectively. Long-axis and short-axis scans should be performed to detect tendinopathy and evidence of bursitis between the tendons and trochanter.
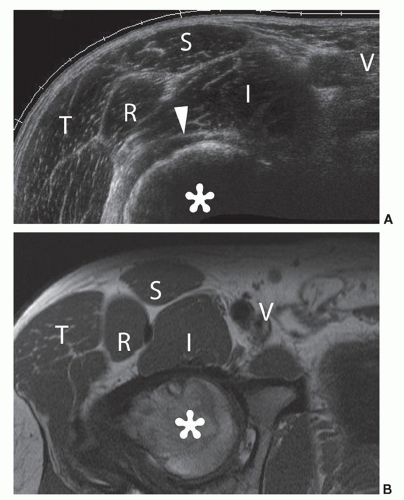
Evaluation of the hamstring origins is performed with the patient prone with the feet hanging over the end of the couch. Lower frequency probes may be required for larger patients. The ischial tuberosity is first identified, located medially at the junction of buttock and thigh (Fig. 6.5). The origin of the ischiocrural tendons (semimembranosus, long head of biceps femoris, and semitendinosus) attaches laterally (Fig. 6.6). They cannot always be appreciated separately at this level on
ultrasound, but individual tendons can be identified by tracing them caudally. They initially separate into the semimembranosus tendon (medial) and the conjoined tendon of semitendinosus and the long head of biceps femoris (superficial and lateral). The conjoined tendon then further divides into the muscle bellies of semitendinosus (medial) and biceps femoris (lateral). Evaluate them in longitudinal and transverse planes to assess for tendinopathy (Fig. 6.7). Lateral to the ischiocrural tendon origin, the sciatic nerve has the characteristic neural fascicular pattern (Fig. 6.6).
ultrasound, but individual tendons can be identified by tracing them caudally. They initially separate into the semimembranosus tendon (medial) and the conjoined tendon of semitendinosus and the long head of biceps femoris (superficial and lateral). The conjoined tendon then further divides into the muscle bellies of semitendinosus (medial) and biceps femoris (lateral). Evaluate them in longitudinal and transverse planes to assess for tendinopathy (Fig. 6.7). Lateral to the ischiocrural tendon origin, the sciatic nerve has the characteristic neural fascicular pattern (Fig. 6.6).
Medial
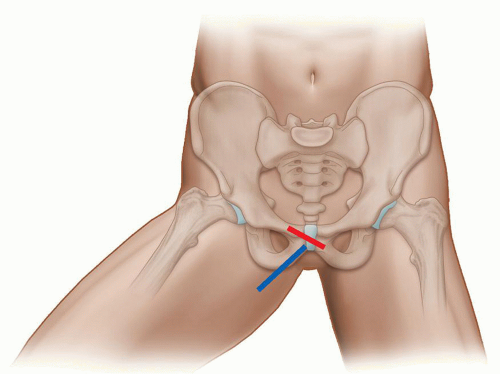
This quadrant contains the origins of the adductor muscles: longus, brevis, and magnus along with gracilis and the insertion of iliopsoas. The patient is scanned in the supine position with the thigh abducted and externally rotated and the knee flexed (Fig. 6.8). The insertion of iliopsoas can be best assessed in this position using LS views, as anisotropy is a common problem, with the leg in the adducted position.
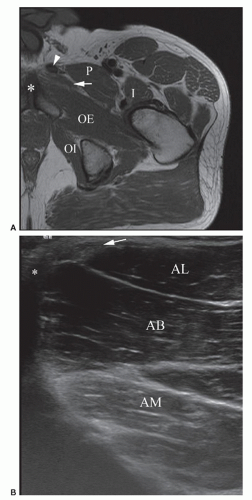
Place the probe over the anterior aspect of the medial muscle compartment, medial to the neurovascular bundle to reveal the three adductor muscles separated by hyperechoic fascial planes (Fig. 6.9). Adductor longus is anterior, adductor brevis is intermediate, and adductor magnus lies posterior. Gracilis is found medial to adductor longus and superficial to adductor brevis. The muscles can be traced to their origin at the pubis to evaluate for tendinopathy and muscle tears using LS and TS views. Next, assess the
symphysis pubis joint space, found medial to the adductor insertion, for arthropathic changes (Fig. 6.10).
symphysis pubis joint space, found medial to the adductor insertion, for arthropathic changes (Fig. 6.10).
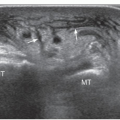
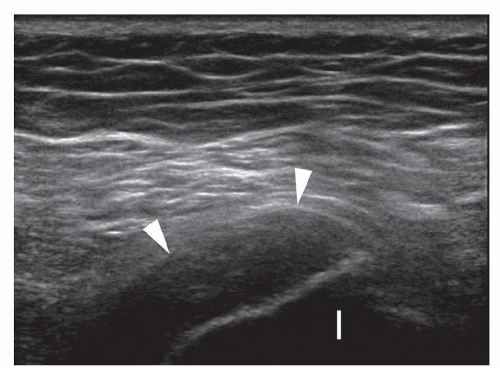 Figure 6.7. Overuse hamstring tendinopathy. Longitudinal sonogram shows hypoechoic thickened hamstring origin tendons (arrowheads) and adjacent ischial tuberosity (I). |
HIP JOINT EFFUSION
Effusions of the hip joint are almost always pathological, but are rarely detectable clinically owing to the depth of the hip joint. Ultrasound is rapid, portable, and effective1,2,3, avoids unnecessary “dry” aspirations and joint contamination, and shows extra-articular collections. Power Doppler identifies coexisting inflammation. Ultrasound can be employed to guide diagnostic aspiration, biopsy, and therapeutic injections.4
Causes
Hematological seeding during bacteremia is the most common cause of septic arthritis, most often due to Staphylococcus aureus.3 Risk factors include IV drug abuse, endocarditis, indwelling catheters, advanced age, immunosuppression, and preexisting joint injuries.5 Other causes include adjacent surgery or direct extension of infection from the abdomen via iliopsoas. Rapid diagnosis is vital as delay worsens the prognosis.3 Bacterial arthritis results in irreversible loss of joint function in 25% to 50% of patients and 5% to 15% fatality rates.6 The appearances of the effusion in terms of complexity and echogenicity do not predict infectious or inflammatory nature.7 Ultrasound cannot prove sepsis and if suspected, analysis of aspirated fluid is usually required. However, large joint effusions with extra-articular extension in painful prosthetic hips are strongly associated with infection.8
Inflammatory arthritides, both seropositive and seronegative, cause hip effusions. Immunosuppressive treatment increases the risk of avascular necrosis and infection, which themselves cause effusions. Increased synovial power Doppler flow suggests an inflammatory rather than noninflammatory cause, but does not differentiate between types of inflammation, for example, infective or noninfective.9 Marginal erosions may rarely be seen at the bone-cartilage interface at the femoral head and neck in inflammatory arthropathy.
Benign tumors of bone and soft tissue, including synovial osteochondromatosis, pigmented villonodular synovitis, osteoid osteoma, and giant cell tumors of bone can be associated with hip effusions.10 Effusions in association with malignant tumors of bone may indicate joint involvement or be a consequence of underlying pathological fracture, but MRI and plain radiographs provide the optimum method of assessment.
Effusions can occur in osteoarthritis. Synovitis and debris in the joint and underlying osteonecrosis may occur.10 Osteophytes at the femoral head/neck junction can also be demonstrated, but are better shown on plain radiographs, computed tomography (CT), and MRI.1
For clinically suspected fracture not confirmed by radiographs, MRI is the investigation of choice. Ultrasound is not very sensitive or specific in these cases, but may occasionally show a femoral neck fracture. In addition to the resulting hip effusion/hemarthrosis, the fracture may be seen as a step or breach of the hyperechoic cortex.1 An osteochondral injury could also result in effusion, but would not itself be visible on ultrasound.
Postsurgical complications such as infection, aseptic loosening, or wear debris can all result in effusion. Ultrasound appearances of hip replacement will be discussed later in the chapter.
Identifying Pathology on Ultrasound
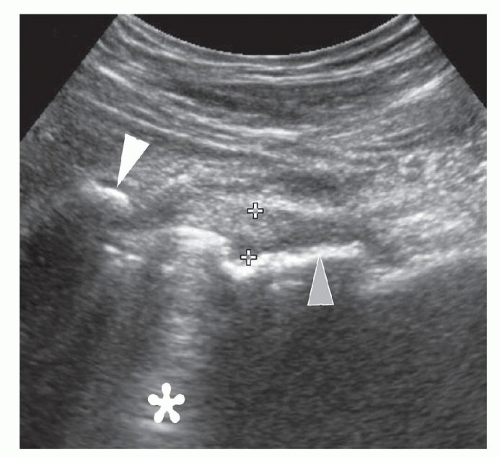
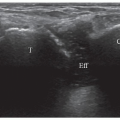
An effusion appears as a hypoechoic layer between the hyperechoic bone cortex of the femoral neck and the
overlying psoas muscle, extending along the anterior recess on the femoral neck (Fig. 6.11). The optimal probe position is an oblique longitudinal scan along the femoral neck. Hip joint effusions as small as 1 to 2 mL may be identified,11 although small effusions may “disappear” into the posterior recess and become undetectable on ultrasound.
overlying psoas muscle, extending along the anterior recess on the femoral neck (Fig. 6.11). The optimal probe position is an oblique longitudinal scan along the femoral neck. Hip joint effusions as small as 1 to 2 mL may be identified,11 although small effusions may “disappear” into the posterior recess and become undetectable on ultrasound.
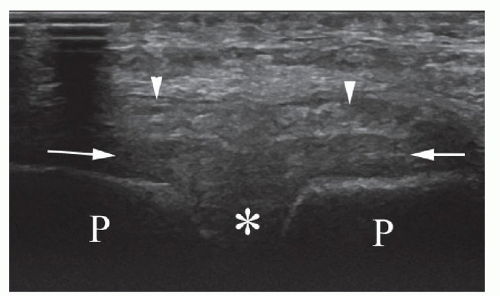
Effusion can be difficult to distinguish from the synovial thickening. Under normal circumstances, synovium is not visible on ultrasound, and the fibrous layer is a thin hyperechoic band (Fig. 6.11). Aging, degenerative disease, synovitis, and surgery can result in thickening of the synovial layer or joint capsule, and this may be mistaken for an effusion (Fig. 6.12).
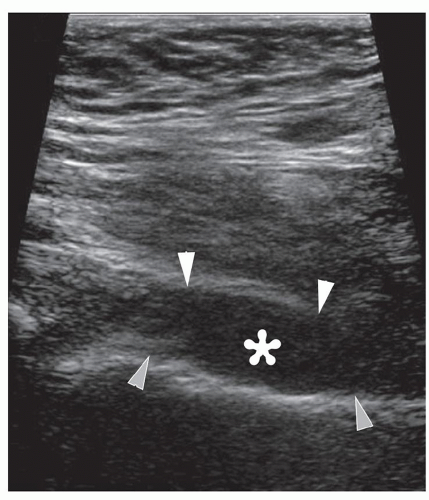 Figure 6.11. Hip effusion. Longitudinal sonogram shows anechoic hip effusion (asterisk) between the hyperechoic capsule (white arrowheads) and femoral neck (gray arrowheads). |
Tips For Identifying an Effusion:
Ensure the probe is perpendicular to avoid anisotropy artifact.
Check the joint contour—a convex, bulging contour suggests effusion.
Do not press too firmly with the probe or small effusions may be effaced.
Compare with the other hip. A discrepancy of 1 to 2mm or more has been described as significant,12,13 but this needs to be interpreted with caution as surgery, aging, and previous synovitis may leave residual capsular thickening.10
Use power Doppler: the presence of internal flow indicates thickened synovium rather than fluid.
If still in doubt, a diagnostic tap can be performed to confirm the presence of fluid, with the advantage that a sample is then available for biochemical and microbiological analysis.
It is important to be aware that other pathologies may mimic effusions on USS.
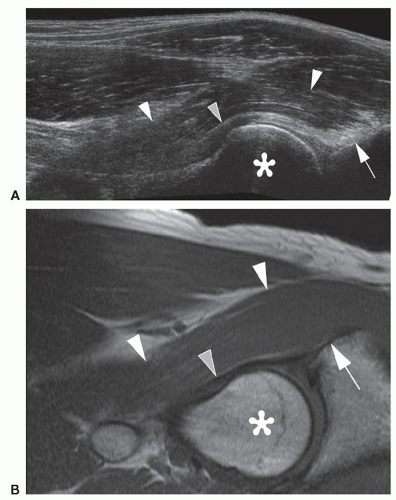
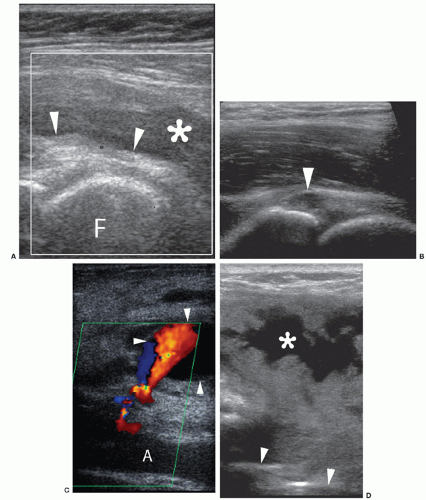
The iliopsoas bursa lies between the hip joint capsule and the iliopsoas muscle/tendon. The bursa is usually empty and not normally visible, but appears as an anechoic/hypoechoic area anterior to the capsule if bursitis is present (Fig 6.13A).
Paralabral cysts can form near the anterior recess (Fig 6.13B).
Femoral artery aneurysms are identifiable by their pulsatile nature: check with Doppler for internal flow (Fig 6.13C).
Extra-articular abscesses/collections do not communicate with the joint and may demonstrate surrounding increased Doppler flow (Fig 6.13D).
ASPIRATION AND INJECTION
Aspiration can show if bacteria or crystals are present and can relieve pain by decompressing large effusions. Ultrasound-guided aspirations reduce the risks of a “dry tap” or contamination of a sterile hip with the infected contents of an extra-articular collection compared with blind aspirations that rely on bony landmarks. The absence of ionizing radiation makes it preferable in pregnant women and children when compared to fluoroscopy or CT. A major concern when performing hip aspiration is to avoid iatrogenic septic arthritis. The risk is low (<1/1,000) provided that proper aseptic technique is observed,14 but this should be discussed when obtaining consent from the patient. Ultrasound-guided joint injections can be diagnostic (contrast for arthrography, including MR arthrography) and diagnostic/therapeutic (local anesthetic and steroid for arthritis). Recent evidence has suggested that local anesthetic may be chondrotoxic and should be used with caution.15 This is also discussed in Chapter 14 Interventional.
There are two methods for aspirating an effusion/suspected effusion from the hip. The first is to use ultrasound to mark the position of the target point on the skin, and then proceed “blind.” The second method is to place the needle and aspirate under direct ultrasound vision. For both techniques, strict aseptic technique is essential. A sterile probe cover should be used for the direct vision method. A spinal needle of at least 18G caliber should be chosen, as pus may be thick and viscous, and a narrow needle may result in a “dry tap.” The needle length should be at least 5 cm, but may need to be longer, depending on patient size. Pre-measurement of the skin-to-effusion distance with ultrasound is useful if in doubt. Infiltration with 1% lidocaine is advised for local anesthesia. Care should be taken to avoid the neurovascular structures medially.
Tip:
If the first aspiration attempt is unsuccessful, rotate the bevel clockwise or counterclockwise. If the needle becomes blocked with debris, reinsert the stylet, and this may resolve the problem. Gradual needle withdrawal while applying gentle suction may succeed where the bevel has become embedded in the periosteum. Viscous or small effusions can be difficult to aspirate, and in these cases injection of a small volume of sterile saline into the effusion may aid aspiration.7 This is particularly useful in cases with a high clinical suspicion of infection but minimal effusion.
Blind aspiration offers the advantage of a quick procedure for anxious/agitated patients and a simpler needle approach for deep joints in large patients. The probe is orientated along the femoral neck in longitudinal and/or transverse section, with the center of the footprint at the desired point of aspiration. The ends of the footprint are marked, followed by the center point, once the probe has been removed. Alternatively, the skin should be marked with the transducer oriented along both short and long axes of the femoral neck and the needle inserted at the point of intersection of the two lines. Following aseptic preparation and injection of local anesthetic, the needle is advanced through the skin vertically down to the femoral cortex, and aspiration is then performed. It is essential that the needle is vertically oriented.
The direct vision technique is useful for small effusions that can be difficult to target and for injections so that the position of the injectate can be confirmed. Transverse or longitudinal probe orientation is used with the transducer centered over the effusion. The end of the transducer is marked on the skin. After cleaning the skin and injecting local anesthetic, the needle is advanced obliquely under direct ultrasound guidance (Fig. 6.14). The needle should be inserted parallel or as close to parallel as possible to ensure maximum visualization of the needle tip. This may be difficult in larger patients, and altering transducer angulation or employing beam steering may help.
Tip:
If difficulty in needle visualization occurs, first check the alignment of the probe and needle, and reposition as necessary. Short, gentle needle motions can help reveal the location of the tip.
Magnetic resonance imaging provides additional information regarding the surrounding bone, cartilage, and peri-articular soft tissues compared with ultrasound. This is particularly useful in suspected infection, tumor, arthritides, and occult fracture. Small effusions that recede into the posterior recess in the supine position may also be more readily detected by MRI. The advantages of ultrasound as an initial test, particularly in suspected infections when speed is of the essence, are that it is quick, readily available, and offers diagnostic and therapeutic intervention.
TOTAL HIP REPLACEMENT
Sonographic imaging in the early postoperative period is a challenge due to difficulties recognizing “normal” postoperative changes from evolving pathologies such as major hematoma and associated infection. Clinical detection of these pathologies is often difficult, and ultrasound has been advocated16, 17 as having superior sensitivity to clinical examination18 and avoiding the difficulties posed by susceptibility artifact on MRI. Ultrasound demonstrates extra-articular collections not shown on arthrography. Where mobility is poor, ultrasound offers portable ward-based assessment. In the late postoperative period, ultrasound provides dynamic assessment of associated pathology such as gluteus minimus or medius tendon tears. Ultrasound can also guide aspiration of joint or fluid collections, capsular biopsy, and therapeutic injection.
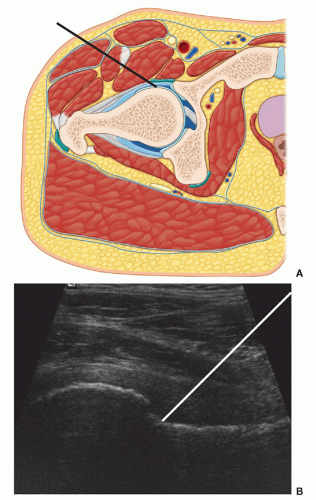 Figure 6.14. Approaches to hip aspiration. The two approaches to aspirating a hip effusion are demonstrated. A: The transverse approach (black line). B: The longitudinal approach (white line). |
Normal Postoperative Appearances
In the early postoperative period, a hypoechoic track extending from the skin incision to the hip represents
the path of surgical access. Collections may routinely be identified along this path or around the prosthetic joint or the femur. The surface of the prosthesis is highly echogenic, more so than normal bone cortex, and may generate reverberation artifact (Fig. 6.12). A study of 47 postoperative hips at the second and fifth postoperative day concluded that a bone-to-capsule thickness of up to 6 mm, deep soft tissue fluid collections up to 21 mm, and superficial collections up to 28 mm were all considered to be normal.16 Communication with the joint is an important distinction that is usually demonstrable by ultrasound, though it may be more readily demonstrated by arthrography.19
the path of surgical access. Collections may routinely be identified along this path or around the prosthetic joint or the femur. The surface of the prosthesis is highly echogenic, more so than normal bone cortex, and may generate reverberation artifact (Fig. 6.12). A study of 47 postoperative hips at the second and fifth postoperative day concluded that a bone-to-capsule thickness of up to 6 mm, deep soft tissue fluid collections up to 21 mm, and superficial collections up to 28 mm were all considered to be normal.16 Communication with the joint is an important distinction that is usually demonstrable by ultrasound, though it may be more readily demonstrated by arthrography.19
Subsequently, thickening of the joint capsule may be seen due to fibrosis and previous hemorrhage10 (Fig. 6.12). Effusions and extra-articular collections are not normal after the first few weeks (Fig. 6.13D).
Complications
Mechanical loosening of the prosthesis is a common cause of pain after total hip arthroplasty. Infection is less frequent, but more serious. Preoperative diagnosis is important because prosthetic loosening is treated by revision arthroplasty, while infection often requires removal of the prosthesis followed by multistage revision procedures8 and prolonged courses of antibiotics. Infection may occur years after arthroplasty, but typically occurs within 2 years of the initial operation, and may be due to hematological seeding from a remote site,20,21 for example, urinary tract infection.
Distinguishing between loosening and infection using clinical assessment and radiographs alone is often not possible. Magnetic resonance imaging is limited by the artifact even when metal artifact reduction sequences are used, although large collections can be demonstrated. Ultrasound can identify and characterize fluid collections and effusions (Fig. 6.15). A study of 48 patients with total hip replacement (THR) found that infected hips have intra-articular fluid and fluid collections outside the pseudocapsule in most cases.8 Guided aspiration can be performed in these cases to confirm or exclude infection. Prosthetic wear can also cause effusions in patients with replacement joints.8
Tip:
If uncertainty exists regarding the communication between collection and joint, do not use the same needle to aspirate or penetrate both in the same pass, as infection may be disseminated between the two.
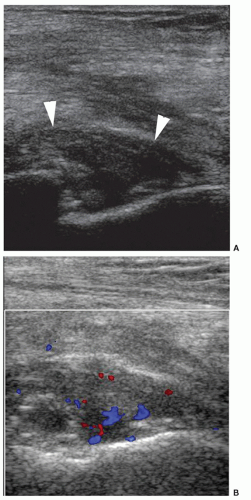
Communications between the bursae of the greater trochanter, iliopsoas bursa, and supra-acetabular region are common in patients with pain following hip arthroplasty22 (Fig. 6.16). Bursitis can occur as a result of impingement of the prosthesis on the surrounding soft tissues, especially the acetabular cup; for example, bony spurs or cement may impinge on the rectus femoris tendon. Guided injection can offer diagnostic/therapeutic benefit in these cases. Care should be taken because of the increased susceptibility of prosthetic hips to iatrogenic infection.
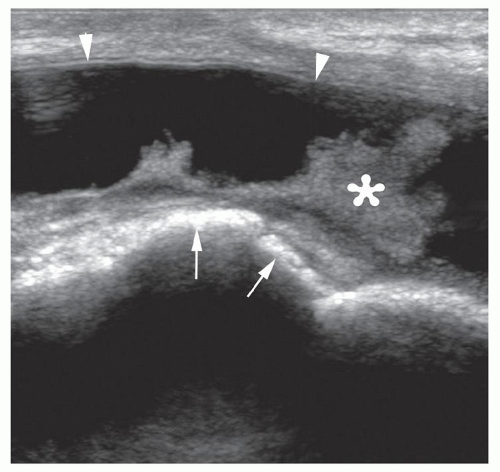 Figure 6.16. Bursa following THR. Longitudinal sonogram shows large anechoic bursa (arrowheads) with fronds of synovial thickening (asterisk) against hyperechoic prosthetic hip (arrows). |
Hematoma is a common postoperative finding. Major hematomas can cause shock and increase the risk of infection. Appearances vary with the age of the hematoma, but are typically mixed echogenicity with a characteristic web-like appearance (Fig. 6.17). Slow swirling may be seen within a hematoma. Significant color flow indicates active bleeding, and arterial trace indicates a false aneurysm (Fig. 6.13C). In cases of non-active hematoma, ultrasound may further assist by therapeutic aspiration or marking for surgical drain placement.
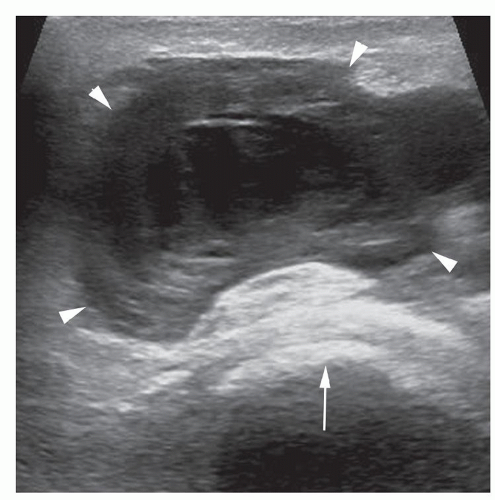 Figure 6.17. Organizing hematoma following THR surgery. Transverse sonogram shows organizing hematoma (arrowheads) with complex internal structure and underlying hyperechoic prosthesis (arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|