Multidetector computed tomographic (CT) angiography is rapidly becoming a pivotal examination in the initial evaluation of patients with hemorrhagic stroke. This article provides an update of the literature on this dynamic topic, focusing on (1) the utility of CT angiography in the identification of hemorrhagic stroke patients who harbor an underlying vascular etiology and the role of the secondary intracerebral hemorrhage score, as well as (2) the clinical value of the CT angiography spot sign and spot sign score in patients with primary intracerebral hemorrhage.
According to the National Stroke Association 2009 Fact Sheet, hemorrhagic stroke accounts for 13% of cases of acute stroke in the United States, with approximately 100,000 hospital admissions per year. Hemorrhagic stroke has a worse prognosis than ischemic stroke, with up to 50% 30-day mortality and very high rates of severe neurological disability among survivors. There are 2 major types of hemorrhagic stroke: (1) those that are due to an underlying vascular lesion such as an arteriovenous malformation (AVM), aneurysm with intraparenchymal rupture, dural venous sinus (or cerebral vein) thrombosis (DVST), vasculitis, and Moya-Moya disease, which represent a minority of cases and are potentially treatable (secondary intracerebral hemorrhage [ICH]); and (2) those that are not due to an underlying vascular lesion (primary ICH).
Timely and accurate identification of patients with secondary ICH is important because these patients may benefit from prompt surgical or endovascular intervention once an underlying vascular abnormality has been identified, since rates of rehemorrhage, with increased morbidity and mortality, can be as high as 18% per year. Although conventional catheter angiography remains the gold standard for the detection of underlying vascular etiologies in patients with hemorrhagic stroke, thanks to its widespread availability, rapidity of acquisition, lower cost, and favorable risk profile, multidetector CT angiography (MDCTA) is rapidly becoming the favored diagnostic tool in the initial evaluation of this patient population.
For patients with primary ICH, the total volume of extravasated intracranial blood is the most potent predictor of mortality and poor outcome among survivors, and those patients who develop hematoma expansion after admission have a worse prognosis than those who do not. Although current treatment options for primary ICH consist primarily of supportive measures, emerging hemostatic therapies aiming to reduce the extent of hematoma expansion such as recombinant activated factor VII or intensive blood pressure reduction may be able to decrease the dreadful morbidity and mortality of this disease. Nevertheless, given that most patients with primary ICH do not experience hematoma expansion after admission, accurate patient selection to target hemostatic therapy only to those patients who are actively bleeding at the time of presentation—and hence are most likely to benefit from hemostatic therapy—is imperative.
Importantly, several recent studies with large cohorts of acute hemorrhagic and ischemic stroke patients evaluated with MDCTA on admission, have found that the incidence of acute contrast-induced nephropathy in this patient population is low, ranging from 2% to 7%, and that this risk is not higher in patients whose baseline creatinine value is unknown at the time of scanning.
Accuracy of multidetector CT angiography for the detection of underlying vascular lesions in hemorrhagic stroke
Several recent studies comparing the accuracy of MDCTA to the gold standards of conventional catheter angiography as well as findings of surgical hematoma evacuation and autopsy have demonstrated that MDCTA can accurately identify those patients with hemorrhagic stroke who have an underlying vascular etiology (secondary ICH), with sensitivities ranging from 89% to 96% and overall accuracy rates of 91% to 99% ( Table 1 ). Thus, MDCTA is an accurate diagnostic tool that allows for the rapid identification of the important minority of patients with hemorrhagic stroke who have secondary ICH, which, in turn, allows for the prompt institution of endovascular or surgical treatment in suitable patients in order to prevent rebleeding from the causative vascular lesion.
| Study | N | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| Yeung et al | 55 | 89 | 92 | 91 |
| Romero et al ,a | 43 | 96 | 100 | 98 |
| Yoon et al | 78 | 96 | 100 | 99 |
| Delgado Almandoz et al ,a | 210 | 96 | 98 | 98 |
a These studies also included findings of surgical evacuation and autopsy in addition to catheter angiography as gold standards.
Frequency of Secondary ICH in Patients with Hemorrhagic Stroke Evaluated with MDCTA
The incidence of underlying vascular etiologies for an ICH varies significantly according to the patient’s clinical characteristics and noncontrast CT (NCCT) findings, with patient age being one of the most important variables. Indeed, in recent MDCTA studies the frequency of secondary ICH has ranged from 13% to 28% in patients older than 18 years to 65% in patients 40 years or younger ( Table 2 ). Table 3 provides a summary of the different types and relative frequencies of causative vascular lesions in a cohort of 845 ICH patients evaluated with MDCTA at the authors’ institution over a 10-year period.
| Study | N | Age Range (Years) | ICH Location | Secondary ICH (%) |
|---|---|---|---|---|
| Gazzola et al ,a | 96 | 16–86 | All | 25.0 |
| Romero et al ,b | 43 | 4–40 | All | 65.1 |
| Yoon et al ,a | 78 | 21–86 | Lobar | 28.2 |
| Delgado Almandoz et al ,b | 623 | 18–94 | All | 14.6 |
| Delgado Almandoz et al ,b | 222 | 18–94 | All | 13.1 |
a These studies excluded patients with primarily/predominantly subarachnoid hemorrhage.
b These studies excluded patients with any subarachnoid hemorrhage in the basal cisterns.
| Vascular Etiology | N | % |
|---|---|---|
| Arteriovenous malformation | 55 | 45.8 |
| Aneurysm with purely intraparenchymal rupture | 26 a | 21.7 |
| Dural venous sinus/cortical vein thrombosis | 20 b | 16.7 |
| Arteriovenous fistula | 11 | 9.2 |
| Vasculopathy | 4 c | 3.3 |
| Moya-Moya | 4 | 3.3 |
b Includes 2 cases of isolated cortical vein thrombosis.
c Includes a patient in whom vasculitis led to pseudoaneurysm formation and rupture.
Practical Risk Stratification for the Presence of Underlying Vascular Lesions in Patients with Hemorrhagic Stroke: The Secondary ICH Score
In a recent series of 623 consecutive patients who presented with hemorrhagic stroke at the authors’ institution over a 9-year period, several independent clinical and NCCT predictors of a higher incidence of a causative vascular lesion were identified in ICH patients evaluated with MDCTA: (1) age younger than 46 years (47%), lobar (20%) or infratentorial (16%) ICH location, female sex (18%), and absence of known hypertension or impaired coagulation at presentation (33%).
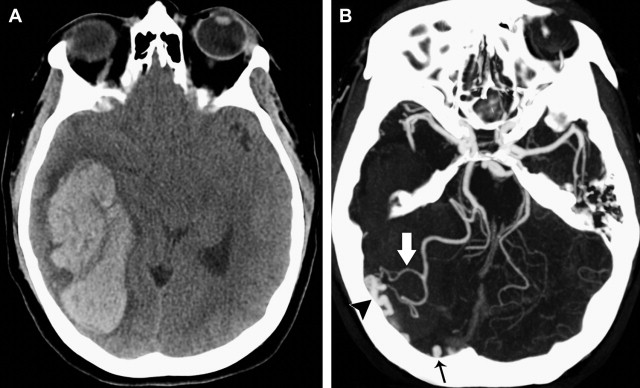
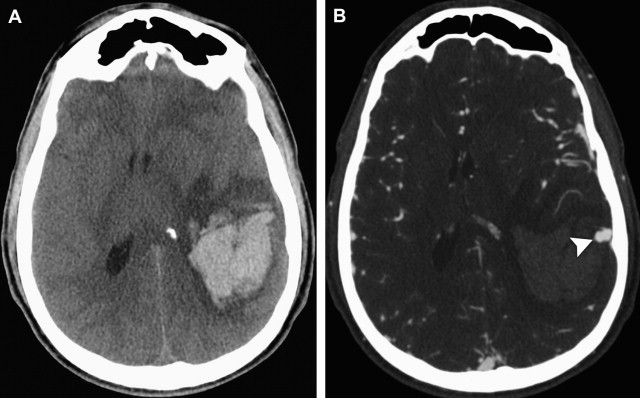
In addition, the authors devised a system to categorize the NCCT according to the probability that an underlying vascular etiology would be found on the subsequent MDCTA examination. In this system, a high-probability NCCT was defined as an examination in which there were either (1) enlarged vessels or calcifications along the margins of the ICH, or (2) hyperdensity within a dural venous sinus or cortical vein along the presumed venous drainage path of the ICH. A low-probability NCCT was defined as an examination in which (1) none of the findings of a high-probability NCCT were present, and (2) the ICH was located within the basal ganglia, thalamus, or brainstem. An indeterminate NCCT was defined as an examination that did not meet criteria for a high- or low-probability NCCT (most commonly, lobar or cerebellar ICH). Of note, in multivariate analysis the authors found that the NCCT categorization according to this system superseded ICH location (defined as lobar, cerebellar, or deep gray matter) as a predictor of an underlying vascular etiology for the ICH.
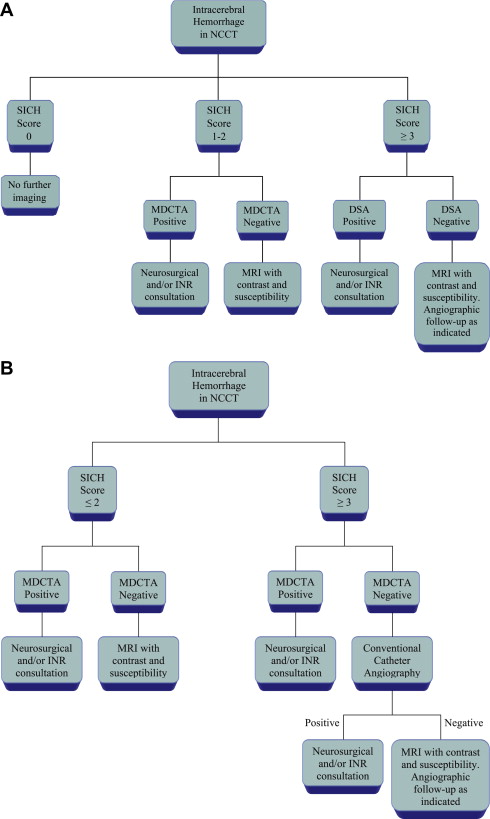
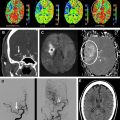
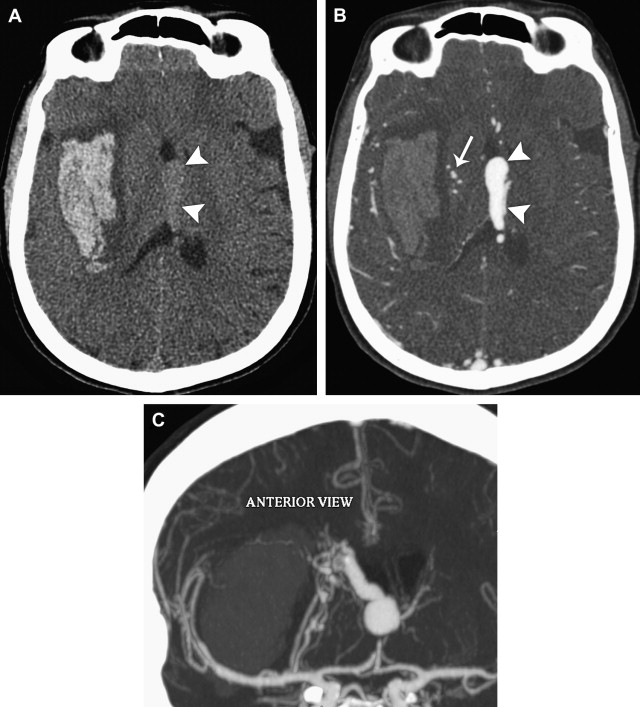
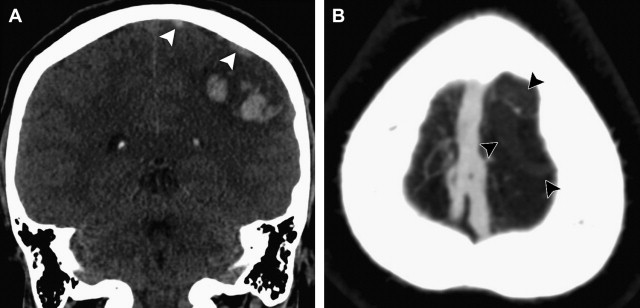
Although a hemorrhagic stroke patient presenting with any of the aforementioned clinical or NCCT characteristics would be at a significantly increased risk of having secondary ICH, the clinical scenario encountered is often complex. For example, one may be presented with a male patient younger than 46 years who has a history of hypertension and presents with a basal ganglia hemorrhage; or, alternatively, a female patient in her 50s or 60s who has a history of hypertension and impaired coagulation but presents with a lobar ICH ( Fig. 1 ). Hence, a scoring system that integrates a given hemorrhagic stroke patient’s risk of harboring an underlying vascular etiology for the ICH would be desirable and clinically valuable. The authors have recently devised such a scoring system based on the aforementioned independent predictors of a higher incidence of secondary ICH, utilizing the NCCT categorization rather than ICH location, and have designated it the Secondary ICH (SICH) Score ( Table 4 ). Table 5 provides the predictive value of the SICH score in the retrospective derivation cohort of 623 patients as well as in a prospective validation cohort of 222 patients. Figs. 2, 3 , and 4 depict patients with high SICH scores and secondary ICH demonstrated by MDCTA.
| Parameter | Points |
|---|---|
| NCCT categorization a High probability Indeterminate Low probability | 2 1 0 |
| Age group (years) 18–45 46–70 ≥71 | 2 1 0 |
| Sex Female Male | 1 0 |
| Neither known HTN nor impaired coagulation b Yes No | 1 0 |
a High-probability NCCT: an examination with either (1) enlarged vessels or calcifications along the margins of the ICH, or (2) hyperdensity within a dural venous sinus or cortical vein along the presumed venous drainage path of the ICH. Low-probability NCCT: an examination in which neither (1) nor (2) are present and the ICH is located in the basal ganglia, thalamus, or brainstem. Indeterminate NCCT: an examination that does not meet criteria for a high- or low-probability NCCT.
b Impaired coagulation defined as admission INR >3, aPTT >80 seconds, platelet count <50,000, or daily antiplatelet therapy.
| Retrospective Cohort (N = 623) | Prospective Cohort (N = 222) | All Patients (N = 845) | ||||
|---|---|---|---|---|---|---|
| Score | N (%) | % Positive CTAs | N (%) | % Positive CTAs | N (%) | % Positive CTAs |
| 0 | 37 (5.9) | 0 | 15 (6.8) | 0 | 52 (6.1) | 0 |
| 1 | 145 (23.3) | 1.4 | 67 (30.2) | 1.5 | 212 (25.1) | 1.4 |
| 2 | 209 (33.5) | 5.3 | 68 (30.6) | 4.4 | 277 (32.8) | 5.1 |
| 3 | 138 (22.2) | 18.1 | 40 (18.0) | 20 | 178 (21.1) | 18.5 |
| 4 | 61 (9.8) | 39.3 | 21 (9.5) | 38.1 | 82 (9.7) | 39 |
| 5 | 28 (4.5) | 85.7 | 10 (4.5) | 80 | 38 (4.5) | 84.2 |
| 6 | 5 (0.8) | 100 | 1 (0.4) | 100 | 6 (0.7) | 100 |
| AUC (95% CI) | 0.86 (0.83–0.89) | 0.87 (0.82–0.91) | 0.87 (0.84–0.89) | |||
| MOP | >2 | >2 | >2 | |||
| Sensitivity | 85.7 | 86.2 | 85.8 | |||
| Specificity | 71.1 | 75.6 | 72.3 | |||
| P value | <.0001 | <.0001 | <.0001 | |||
The SICH score is advantageous because it can be rapidly calculated immediately after the NCCT examination has been performed, while the patient is still on the CT scanner table, and thus serves as a valuable tool in the clinical decision as to whether to perform MDCTA. Indeed, this scoring system would be most useful at institutions where neurovascular imaging is not performed in all ICH patients but is reserved for those patients who are deemed most likely to harbor an underlying vascular abnormality. However, given the relatively high positive rate of MDCTA for the presence of an underlying vascular etiology in patients with hemorrhagic stroke (14.2% in the authors’ cohort), some institutions (including the authors’) perform MDCTA in all patients with this condition. Hence, the usefulness of the SICH score at the latter institutions lies in selecting ICH patients for more invasive diagnostic tests such as conventional catheter angiography when the initial MDCTA is either negative or inconclusive but the patient’s pretest probability of harboring an underlying vascular lesion—as determined by the SICH score—is high. Of importance, results of receiver operating characteristic (ROC) analysis in the authors’ patient cohort showed that the maximum operating point was reached at a SICH score of greater than 2. Fig. 5 provides proposed algorithms for the diagnostic workup of patients with hemorrhagic stroke based on the admission SICH score at institutions that perform neurovascular imaging selectively (see Fig. 5 A) as well as at institutions that perform MDCTA in all patients (see Fig. 5 B).