Rupsa Bhattacharjee, Prashant Nair, Jaladhar Neelavalli, Indrajit Saha Advances in technology & in our understanding of Illness and Disease together With an expanded workforce & greater resources will allow us to provide more services to a higher quality. John Hutton Commercial availability of MRI (magnetic resonance imaging) started in the 1980s, and soon it became a strong nonionizing investigating tool. Since then, MRI has taken big leap in terms of advancement and improvement. Researchers are working day in day out to deliver better, faster and crisper images using different permutation combination of spin echo (SE), gradient-recalled echo (GRE), echo-planar imaging (EPI) and k-space filling-based techniques with more detail in every technology introduced and being introduced in future. MRI is used clinically to generate images overall anatomy of a human body. However, the trade-off is that MRI generally takes longer time in scanning and forming images. It is especially long compared with ultrasound or CT. Depending on the area and type of scan, it might take few minutes to an hour or so. The long scan time results into patient discomfort, which is then manifested as patient movement within the scanner, thereby generating artefacts. There are typical anatomical structures such as heart, or capturing typical contrasts such as flowing blood, which require faster acquisition time. Thereby the MR researchers are always looking for acquisition techniques, faster and reliable. Parallel imaging thus comes into the picture in rescue. This is a certain way of MRI scan that hastens the scan by reducing the acquisition time via robust techniques. It also enables MRI to be applicable in challenging situations, where time constraint is crucial. Parallel imaging technique is one of the faster acquisition techniques which is introduced in 1990, and it further developed and is now known by the name of SENSE/ASSET and GRAPPA/ARC. Basically using PI, faster acquisition is achieved and is of two types: Parallel imaging technique is widely used across by all the MR vendors. The positioning of the receiver coils, as well as the sensitivities, plays an important role to enable how the MRI signal is being localized spatially. These important features associated with the receiver coils allow to reduce the number of steps in phase-encoding direction. Finally, this results in the several-fold reduction in scan time. To reduce the acquisition time, either the k-space data must be collected more quickly or the amount of k-space data collected must be decreased. The SENSE (sensitivity encoding)/ASSET (array coil spatial sensitivity encoding) is the most widely used parallel imaging technique. The crucial piece of information in SENSE is the knowledge of sensitivity maps associated with coils and the elements. Prescan acquisition, done at the very start of the MR study, can collect this information. Unfortunately, this very knowledge and accuracy of sensitivities of the coils and the maps is the biggest drawback of SENSE. Any kind of error in the maps can eventually manifest into unexpected artefacts in the final image, which is being fully reconstructed. A number of factors can negatively influence the accuracy of the maps. Firstly, coil sensitivity profiles are dependent on how the coil is placed with respect to the scanned anatomy. Any small movement occurred by the patient might alter the coil sensitivity. As a result, the images will be artefactual. One way to avoid this error is to recalculate the coil sensitivity maps and use the latest ones for reconstruction. This becomes more challenging in areas such as sinuses/lungs, where signal levels are low by default and also noise levels are higher. There, it might be a problem to calculate the sensitivity map in the first place. SENSE/ASSET may produce aliasing/wrap-around in the phase-encoding direction if the full field of view (FOV) is smaller than the imaged object but SENSE/ASSET provides slightly higher signal-to-noise ratio (SNR) and better image quality as the acceleration factor is increased and also SENSE/ASSET is faster than GRAPPA/ARC because the self-calibration happening in the k-space does not require any additional time penalty. GRAPPA/ARC is a somewhat longer sequence than SENSE/ASSET because it requires extra time for the self-calibration of k-space lines. GRAPPA/ARC is better for regions which are inhomogeneous in nature, for example, abdomen/lung. As discussed, getting perfectly accurate sensitivity maps for each coil is challenging, which is a disadvantage for SENSE. On the other hand, in GRAPPA, the process of estimating missing k-lines is broadly global. This involves all the coils as well as the ACS region. At the same time, it is unaffected to inhomogeneities that are local. To improve the SNR and estimate accuracy, extra lines can be acquired at the k-space center. Also in single-shot echo planar imaging, GRAPPA/ARC seems better, as the sensitivity maps of the coils are the major drawback. On account of susceptibility effects in EPI images, there are variations in local resonant frequencies, generating distortions. In case of SENSE, the effects of susceptibility, the amount of distortions and the sensitivity maps might be different. Therefore, in case of GRAPPA, the estimation of missing lines in the k-space will have little or no effect to the image distortions. For some typical applications such as cardiac, small FOV MRI images are required as per anatomical area. In such cases, GRAPPA/ARC is supposed to be more convenient. If we reduce the full FOV, the PI process itself induces gross aliasing into the image. In case of SENSE/ASSET, the conventional PI unfolding methods cannot correct the aliasing. This is called “SENSE Ghost” artefact which is usually seen in the centre for the image. On the other hand, GRAPPA/ARC generates full FOV images which are partially aliased already, that too, without modifying the basic reconstruction method. As we know, a small FOV means a greater space between the k-lines than usual. Just increasing line spacing cannot have a major effect on the estimation of missing lines in GRAPPA/ARC. Thereby in case of small FOV applications, GRAPPA/ARC is more preferred. Two major categories of artefacts can arise due to PI: (1) residual aliasing and (2) noise enhancement. The main reason is same for both the artefacts. The acceleration factor being used is quite high compared with the coil geometry capabilities; thus, a huge number of pixels are unfolded from each other. When the coil sensitivity maps are not sufficiently accurate, and also the acceleration factors are quite high, SENSE algorithms face residual aliasing. Residual aliasing can occur, when the patient has moved after the coil sensitivity map scan/prescan. It can be removed by repeating the prescan. Low-signal and high-noise areas also face errors in sensitivity maps. This can be removed by interpolation or smoothening of the sensitivity profile. The issue of noise enhancement happens when separate regions of the coils have similar sensitivity profiles. Such areas will try to stay in the centre of the image. A good number of pixels are unfolded on top of one another in the similar sensitivity regions. So to reduce this kind of artefacts, much research goes into designing coils optimally. Special types of arrays are dedicatedly designed for special type of anatomies, or rather, individual anatomies, i.e., neck coil, cardiac coil, breast coil and spine coil. The SMS (simultaneous multislice)/MultiBand SENSE technique enables simultaneous excitation and acquisition of multiple volumes for multislice single-shot EPI sequences. The simultaneous volume excitation is done using a multiband radiofrequency (RF) pulse. The simultaneously acquired volumes are unfolded by means of the SENSE algorithm. The image unfolding is improved by introducing a linear phase in k-space in the phase direction depending on the volume position. This results in a spatial shift of the aliased pixels in imaging space. MultiBand SENSE allows for shorter repetition times (TRs) with the same in-plane resolution and coverage in the slice direction and consequently leads to shorter scan times. Alternatively, the time savings by the MultiBand acquisition can also be used to keep the TR unchanged and acquire more slices in the same time. To take advantage of parallel imaging acceleration, MB uses coil encoding together with either gradient or RF encoding to resolve data along the slice-select (z)-axis. Because modern coil arrays typically have only a few coil elements in the z-direction, coil sensitivity differences along that axis are rather poor. Accordingly, the simultaneously excited slices must be spaced widely apart (typically at least 25–30 mm) as shown in the diagram. An interleaving technique is then used to excite missing slices in the gaps. In case of fMRI, the required minimum TR is very short. Here, we can use larger FOV in terms of coverage or even higher temporal resolution. With MultiBand SENSE, in DWI/DTI, we can achieve bigger FOV, higher diffusion directions in number, high speed and resolution, all at the same time. Compressed sensing/hypersense (also known as compressive sensing, compressive sampling or sparse sampling) is a signal processing technique built on the fact that signals contain redundant information. Compressed sensing is concerned with the whole chain of acquisition strategy, signal processing and reconstruction. Compressed SENSE combines few of the latest innovations in MR fields such as phased-array coils, existing parallel technology and optimizing speed of reconstruction via automation. Compressed sensing, as a technology, available with many vendors, focusses primarily on the sparsity existing in time domain. However, the “compressed SENSE” takes benefit of sparsity existing in spatial domain. Therefore, this can be suitably applied to multiple anatomies and contrasts, making it more accessible in daily clinical practice. Compressed sensing is often less flexible as a scheme due to its requirement of autocalibration lines. The target is to reach scan acceleration levels almost as par with the SENSE techniques (no involvement of autocalibration lines). Non–SENSE-based compressed sensing methods therefore somewhat adjust the sampling scheme by providing a sample density which is discontinuous. It also leads to low density of sampling in the peripheral areas of k-space. In consequence, the resultant images would be blurry/contain noise that will be structures as well as degrade the image quality. This will be definitely not suitable for most of the anatomies/sequences/contrasts. The implementation of compressed SENSE is totally automated in factors such as creating the sampling pattern or maintaining the balance between constraints of sparsity and consistency. The balanced sampling pattern (SENSE type) uses variable-density incoherently undersampled scheme. It densely samples the center of k-space compared with the peripheral. This also minimizes other artefacts such as patient motion or eddy-current-related issues. The automated balancing act between consistency of data and its sparsity is independent of multiple factors such as anatomy, acceleration factor, receiver coils, image contrast, etc. The major reason of robust and speedy compressed-SENSE reconstruction is due to faster converging processes. This happens by automatically deriving the wavelet threshold. It also helps to reduce the total number of iterations and therefore the overall reconstruction time. Particularly for 3D images, reconstruction compression is optimized. It balances image quality, maintains lower memory capture and allows short reconstructions. From the point of ease-of-use, compressed SENSE is unique, just involving three major parameters to play with. Compressed SENSE helps in the following cases: routine T1, T2, MR angiography, mDIXON-XD, FFE, TFE, spin echo (SE), turbo spin echo (TSE), fat saturation sequences, quantitative methods (mDIXON-Quant, T2 relaxometry), 2D–3D sequences, which can also be used in 4D dynamic scans. Multitransmit or parallel transmission technology is used to achieve homogeneity of the transmitted B1 field. A homogeneous B1 field is generated by transmission of RF energy through independent and multiple RF coil elements to produce their respective B1 subfields experienced by the underlying tissue. The RF coil is divided into multiple coil elements, which are independently powered and parallelly connected and generate circularly polarized fields which enable in mitigating the RF field nonuniformity. In addition to field homogeneity, usage multitransmit technology has advantages of enhanced image contrast and uniformity, reduction in dielectric effects (standing wave artefact) and reduced specific absorption rate (SAR). The first clinical MRI system was introduced in 1984. Since then, attempts have been made to combat issues of RF penetration and associated SAR issues. The concept of multiple receive coils to mitigate these effects was introduced, which was later emerged into parallel imaging techniques. Equivalent to parallel imaging, parallel transmission through multiport excitation for bird-cage coils and concepts of RF shimming were also introduced. All these developments were observed in the timeline of 1984–2008 regarded as the first and second generations of RF technology. These systems mostly focussed on optimizing RF technology and SAR improvements with single RF transmission. Conventional single RF source systems provided B1 excitation field in quadrature mode by splitting the RF source to a body coil. The two signals fed into the orthogonal ports of the coil have a fixed 90 degrees phase shift. Parallel RF transmission technology has individual RF sources independently powering the coil elements. This design provides additional degrees of freedom (relative amplitudes and phases) of the input signals based on underlying anatomy, resulting in enhanced RF homogeneity. The input pulse waveforms from individual RF sources are optimized for phase, amplitude and power through the process of RF shimming before transmission. 4D MultiTransmit – also called RF shimming – provides a more homogeneous B1 field, which translates into a more uniform signal distribution in images and better image contrast. In 3.0 T cardiac MR, MultiTransmit produces images with excellent uniformity. In cine images as well as black blood TSE, this results in better delineation of the myocardium versus the blood pool. Standing wave effects are now significantly reduced, especially beneficial in patients with cardiomyopathies. TrueForm – Conventionally, RF pulses that have a singular amplitude as well as phase are used for MRI. In case of 3 T or above, i.e. high field strengths, the conventional RF pulses might generate B1 inhomogeneities, also manifested as shading artefacts. TimTX TrueForm is the vendor-specific (Siemens) standard solution (introduced in 2007), for this shimming problem with B1. It promises better image quality by using various combination of pulse shape, phase and amplitudes, thereby reducing shading artefacts. This TrueForm was followed by TimTX TrueShape, which is the alternative naming for parallel transmit. TimTX TrueShape allows to shape your RF pulse freely to achieve a new degree of freedom in MRI. This works by the interaction and intelligent communication of multiple transmit channels, which are otherwise independent. It paves the way for innovative applications such as syngo/ZOOMit, which can create a magnifying point of view in MR. This happens because of the following: TimTX TrueShape system consists of two independent RF pulse shapes in the sequence, two independent wave generators along with two RFPAs and two feeding ports at body coil. Therefore the waveforms are fully flexible. A small area can be targeted and excited instead of a large target. The phase-encoding steps involve very small amount of data, making the scan time faster as well as improving the image quality significantly. In case of MultiDrive, independent exciters are dedicated to create various RF pulses with varying shape/waveform/amplitude and phase of pulse. This phase generated is nothing but the time delay between the pulses one other relatively. Every RF pulse goes through the aforementioned independent amplifiers. Next these are applied to the transmit coil, all the four drive ports. MultiDrive works in two specific modes: (1) The “preset mode” makes use of the phase settings and RF, which are precalculated, thereby generating the optimum quality of images applicable to all kinds of patients. The precalculated values/settings are optimally evaluated, taken average of and then applied for a broad range of population. As the name suggests, no prescans are needed for this mode; RF power and SAR required are much less. (2) The second mode is the “per-patient optimization” mode. This works in a selective manner and requires user input to optimize the RF setting for every patient. Accordingly, a quick prescan is calibrated and acquired to create the B1 map for that particular area. Based on the prescan and B1 data, the main RF settings are optimized. If we encounter a challenging patient, whose anatomy differs from the average broader population (obese/ascites patient), this mode might be useful. Multitransmit technology has extensive applications in almost all areas of the body including liver, cardiac, breast, fetal and body imaging. Multitransmit technology can reduce effects of SAR and image nonuniformity with inherent acceleration in acquisition time. Alternatively, the flip angle could also be increased to achieve enhanced SNR. One such cardiac study shows that in a short-axis cine sequence of a fixed flip angle (45 degrees), TR can be either varied over 3.4 to 2.8 ms; or it can be kept fixed at 3.4 ms by varying the flip angle to 58 degrees. A dual-channel transmit coil system has shown a one-third reduction of scan time due to shorter TR values enabled by reduced SAR estimates in a musculoskeletal study. Fetal MRI at 3 T is particularly challenging due to large volumes of high permittivity and conductivity liquid, amniotic fluid being sensitive to transmit field in homogeneities. The added advantages and flexibility of multitransmit systems result in significantly improved image quality. MultiTransmit improves the B1 field being transmitted inside the MR scanner, on the patient, and helps to omit the B1 shading problem. At the same time, improving uniformity in MultiTransmit is closely related to receive shimming. If the transmit B1 is nonuniform field, it reciprocates into nonuniform field of receiving sensitivity as well. The common solution is to use a postprocessing filter (“bias field correction [BiFiC]”) that normalizes the problem. These filters can calculate the correction function from the final image and apply that over the image to regain uniformity. However, these require extensive tuning for each anatomy specifically. Multitransmit improves consistency in images by adapting the RF shimming technique which is patient adaptive. Based on every individual data, the flip angle and RF setting distributions are recalculated. This ensures a consistency over multiple patients as well as contrasts in challenging B1-shading areas (abdomen, breast/body). Digital broadband MR enables MRI signal detection, digitization directly in the RF receive coil. The traditional analogue receive chain in the MRI system is replaced by digital RF chain. dStream architecture has receive coils equipped with miniature Analog to Digital Converter (ADCs) for digitization, and then the signal is transmitted through optical fibres. The MRI signal is sampled directly without conversion to DC through direct digital sampling method. This technique avoids all intermediate analogue stages for downconversion of the signal between coil and the ADC. This novel dStream architecture has advantages such as SNR (signal-to-noise ratio), boost up to 40%, improved signal and phase stability, lower power consumption, improved signal fidelity due to reduction in crosstalk and lossy transmissions as in the case of analog architecture. The dStream architecture replaces the conventional analogue RF chain by the digital RF chain. The MRI signal is sampled directly at the receive coil without conversion to DC using miniaturized ADCs, and the digitized signal is transmitted over optical fibre cables to the reconstructor unit. This technology avoids all intermediate stages of analogue architecture such as multiplexing, switching, filtering of the signal between the coil and the ADC. Since the signals are digitized at the receiving end, the architecture is independent of number of elements/channels of the coil and the system. This makes the architecture flexible and scalable. dStream architecture enables acquisition of signal over the complete dynamic range, thus improving the signal fidelity. Optical fibre transmission provides immunity against crosstalk and interference in addition to simpler architecture, lower power consumption and higher SNR. Improving SNR through dStream technology provides enhanced image quality. This improvement is much appreciated by radiologists and clinicians who are supposed to make decisions for complicated clinical procedures just based on the MR images. Especially in applications such as neuro/MSK/body/cardiac, this is quite crucial. The flexibility of the dStream architecture helps clinics to overcome RF channel scalability and expandability. dStream system provides an overall 40% boost in SNR in whole-body imaging in addition to reduced acquisition time. MRI has the benefit of advanced soft-tissue contrast. If it can be combined with patient setup and treatment plans being revised on MR table itself, that can fully integrate the decision-making process. One such example could be automatizing the beam gating during delivery. This is enabled by cine imaging and structural MR tracking. Similar approach can help in radiotherapy dosage reduction as well. Radiologists can detect lesion areas very clearly, radiation dose being subjected can be altered accordingly. This makes the environment safe and precise as well as effective. The number of sessions of treatment required goes down considerably, which is a boon for such patients. In such cases, the TP (treatment planning) system is totally combined with the treatment delivery software. Both of them share the same database to access patients’ data. Treatment plans are customized for applications such as 3D conformal RT/step-and-shoot intensity-modulated RT. Fluence map optimization combined with leaf sequencing helps in optimizing inverse treatment plan as well. MRgFUS stands for magnetic-resonance-guided focussed ultrasound surgery. This is a noninvasive treatment of tumours, quite for various areas in the liver. MRI-guided focussed ultrasound and FUS (focussed ultrasound surgery) help to treat a wide range of tumours and diseases such as uterine fibroids, desmoid tumours, osteoid osteomas, bone metastases and many more. This technique uses USG transducer with a very high energy and the sound waves to generate heat to the tissue in a very short time (∼20 s). This is an iterative process that goes on repeatedly till the destruction of the target tissue. Role of MR is to plan the area where the treatment is being received and to surveil the amount of real-time heating. Sequential and simultaneous positron emission tomography combined with magnetic resonance imaging (PET/MR) in clinical practice for the need of combining metabolic, functional and anatomical information in a single frame was accomplished as a desirable step in oncologic imaging. MRI operating in whole-body mode and providing the much-desired anatomical landscape of high soft-tissue contrast for whole-body PET has made metastatic workup in cancer more objective. In addition, MRI can offer more options to choose the best imaging plane/s for better display of pathological anatomy impacting surgical planning but with additional imaging time. Whole-body imaging enables screening the entire 3D length of the body to collect information across. If we could employ hybrid modality here, that might improve the image quality and resolution (both spatial ad time). Both sequential and simultaneous imaging modality can be employed to achieve this. Besides, it provides a potential clinical application for multipurpose imaging such as MRI attenuation correction, which is adopted by GE Healthcare. Same couch is used for both the modalities. MRI coils can either or not be used. In case we are using coils during MRI, we should take that off before we proceed for PET. Philips Healthcare introduced the first PET-MR, which includes 3 T whole body with PET and TOF. Sliding nature of the couch was designed to do whole-body imaging in less scan time. The hardware of PET scan are anyway kept outside of MRI, so RF interference/magnet fringe fields should not be cause of any concern here. This gives an advantage of reducing the supply, thereby decreasing the loss of gain in PMT, whereas the simultaneous acquisition modality for whole-body imaging consists of the PET detector with annular rings detectors and 3 T whole-body MRI. PETMR should be a solution for imaging patients with intracranial neoplasms, neurodegeneration, one of the early applications of PETMR has been to better understand dementia and other neurodegenerative diseases. PET and MR images acquired sequentially can be taken off for fusion (as accurate as possible) without PETMR. This capability may limit the adoption of PETMR in neurooncologic applications. Same approach can be taken in neurodegenerative diseases or epilepsy as well. PETMR gives the benefit of scanning patient at the same time for both the purposes conveniently. Therefore, PETMR is a promising modality for oncologic imaging of some regions, such as brain, head and neck, liver and pelvis. Integrated whole-body PETMR hybrid imaging has the following major benefits: At the same time, it has associated scope of improvements such as follows: First simultaneous PETMR detection was demonstrated in 1997, but it took more than decade to develop better detector technologies for clinical systems to become commercially available. This technology combines structural and functional characterization of tissue provided by and PET adds imaging of Physiology and metabolism and tracks labelled cell receptors using 18F-FDG. In conclusion, PETMR is mainly used for across anatomy’s soft tissue detail using different MR sequences and helps in early detection and characterization of cancer diseases. PETMR could enhance the image features with high spatial and time resolution and could be obtained with either sequential or simultaneous imaging modality in PETCT; direct attenuation is possible, but in PET-MRI systems, obtaining attenuation maps is very difficult. PET-MRI uses microstrip transmission line RF coil for attenuation. Manufacturers still have limited microstrip transmission line RF coils to offer; this area requires so much of research to develop PETMR compatible coils. Integration of MR here enables to realize a huge number of separate pulse sequences and associated contrasts within PETMR. Yet there are possibilities to curtail extra imaging sequences to practicality of scenario. Intensity-modulated radiation therapy, volumetric modulated arc therapy or high-dose stereotactic body radiotherapy delivers high doses to the tumour, leaving surrounding tissues and organs at risk. Adding MR into RT becomes most dynamic and fitted imaging modality, and it provides direct visualization of the tumour and surrounding tissue anatomy. Magnetic resonance radiation therapy (MR-RT) has some advantages such as follows: When we combine two outstanding modalities to overcome technological barriers, it offers an amazing result in terms of fast acquisition of high-definition MR imaging and simultaneous radiation delivery. There are majorly four companies that offer MR-LINAC: Elekta (uses 1.5 T MRI), ViewRay MRIdian (uses 0.35 T MRI), The Australian MRI-Linac program (uses 1.0 T MRI) and Magnet Tx Aurora-RT (uses 0.5 T MRI). Every MR-Linac has similar combination – ViewRay MRIdian Linac which integrates a 0.35 T split superconducting magnet with a 6 MV flattening-filter-free (FFF) linear accelerator and similarly Unity System combines high-field 1.5 T Philips Ingenia MR and Elekta Unity 7 MV FFF linear accelerator are fully integrated, provides unmatched magnet field homogeneity and geometric accuracy. High-speed jaws and MLC provide reliable real-time leaf positioning with 1 mm accuracy. RT, although very important in cancer treatment, has two focus points contradictory to each other: MR-Linac is helping to deliver accurate dose with real-time tumour visualization and demarcations. MR-Linac allows to scan, plan and treat in one go. MR gives soft-tissue detail without radiation and helps to get real-time position, shape and size of tumour as we know chances of tumour displacement, size and shape are more when the images taken 1 or 2 days before the therapy. So, without giving tumour a chance to shift or reshape, it allows to plan and treat in real time without overestimating. MR-LINAC is giving confidence and certainty in delivering radiation therapy to the patient who is carrying tumour which changes its shape, size and position anytime. MR-Linac has tremendous potential in personalized precision-guided radiotherapy. MRI-guided adaptive radiotherapy may become a new RT paradigm that allows to offer high-quality and personalized patient care. Opportunities for improvement include high-quality and high-fidelity imaging, better motion management and efficient adaptive workflow as well as biological and functional-based adaption. There are high-field MR-guided surgical procedures which are as minimally invasive as possible. A combined MR suite and operating room (MR-OR) has been developed and extensively assessed for its use in a wide spectrum of therapeutic applications and offered less invasive alternatives for the treatment of lesions in the brain. Standard neurosurgical OR-cum-MR equipment are integrated with both 1.5 and 3 T (60 and 70 cm). Surgeons can have both intraoperative planar and volumetric images of MR. The images would have excellent soft-tissue contrast with high spatial resolution that benefits the planning, guidance and monitoring needed in surgery setups. Morphological MRI, functional and spectroscopy can be done intraoperatively in the same MR-OR. There are developments of perspective surgical navigation device and instruments which can be remotely operated. These will help the surgeons in alignment. If there are any kinds of complications in surgery, that can be addressed immediately. If done in MR surgical approach, then the recurrence rate of the tumour is also proven to be quite less. Intraoperative MRI leads to accuracy improvement, resection assessment, less invasive surgical alternatives and target confirmations (real-time)/therapy delivery in neurosurgery. Multiple approaches to do MR-OR: ceiling-mounted iMRI system specifically for imaging during surgical procedures in a hybrid operating room or in a multiroom hybrid operating suite. This suite has a magnet that is travelling on a rail system which is then mounted to the ceiling. The ceiling lies between the diagnostic room and operating rooms located adjacently. It helps to take the magnet to the patient as quickly as possible. Second method of iMRI is floor-connected with OT table to the MRI table either front side or rear side of the magnet you can slide it to MRI for the scans to be performed. All staff including surgeons with access to the MR-OR suite must undergo an MRI safety class with the MRSO. MRI guidance is increasingly playing an important role in today’s era. At the same time, there is much need to hybridize the MRI-cum-surgical environment. The institutes should be specific on the surgical requirements and financial/physical constraints to finally decide the scanning configuration that is required. MR-RT is an advanced and improved treatment modality used along with chemotherapy in the management of cancer at various disease sites. Around 60% of cancer patients receive radiation therapy during their life span. MR-RT has improved planning and delivery of radiation, leading to superior outcome and reduction in toxicity with better quality of life in cancer patients. There are two methods of doing MR-RT: MR elastography is a noninvasive medical imaging technique to help determine the stiffness of organs in human body. Elastography directs painless low-frequency vibrant into the organs. USG or MRI measures quickly these vibrations moving through the organ. It is commonly used to assess liver fibrosis. For staging liver fibrosis, MR elastography may be a safer, less expensive and relatively more accurate option in comparison with the gold standard liver biopsy. MR-based techniques have been evaluated for assessing hepatic fibrosis, including DWI, perfusion MRI, MR spectroscopy (MRS) and MR elastography. MR elastography is a quantitative MRI-based method to image the direct consequence of fibrosis-associated stiffness of the hepatic parenchyma over larger regions of the liver. MRE is user-friendly than many other ultrasound-based techniques, and the acquisition time of an MRE sequence is less than a minute. Hence, MRE can be a standard abdominal MRI protocol, providing a comprehensive evaluation of the liver, which includes assessment of fat content, presence of focal disease and complications of chronic liver diseases. MRE studies the mechanical properties of the tissues by using mechanical shear waves (60–200 Hz), which propagate more rapidly in stiffer tissue and more slowly in softer tissue. The speed of propagation of waves is reflected in the wavelength, and hence, as tissue stiffness increases, the wavelength becomes longer. Low-frequency mechanical shear waves are generated with a special acoustic driver system and propagated into the body. A modified phase-contrast pulse sequence with cyclic motion encoding gradients synchronized to the mechanical waves is used to image the micron-level displacements associated with wave propagation. The imaging is done in one or more breath-holds, and the image depicts the pattern of propagating waves in the liver. Three steps of MRE are as follows: Future prospective elastography techniques: MR-guided biopsy is a powerful tool to locate or reach the area of interest very accurately and more comfortably without disturbing the nearby vital organs and nerve roots. MR-guided biopsy is a golden standard method to perform the biopsy because of approach the area of interest and sample taken method. Mainly MR-guided biopsy played a major role in two important anatomical structures: (1) breast and (b) prostate. MRI guidance biopsy is comfortable for radiologists with easy planning and to approach the area of interest. MR-guided biopsy has many advancement tools available in different vendors in the market, e.g.. Philips has Dynacad and DynaTrim software methods/packages for preplanning MR-guided procedures. DWI, since its inception, in the 1990s has been used in the diagnosis of early stroke and other neurological disorders. Other contemporary studies during this time and after highlighted its importance in the detection and characterization of oncologic lesions as well. With the advent of recent MRI machines possessing multichannel coils, EPI and stronger gradients application of a diffusion-weighted (DW) sequence in whole-body imaging have gained more popularity. This has led to DWI protocols being performed in less than a minute, with minimal distortion, higher resolution, lesser fat suppression errors and lesser eddy-current-related artefacts. Diffusion is a contrast/weighting in itself and can even save us from certain scenarios requiring contrast infusion. Diffusion and its applications thus find use in oncologic imaging of organs such as liver, prostate gland, breast and whole-body imaging. DWI find its use in oncology, in the way that it helps detect tumours, differentiate tumours, characterize heterogeneity and help in evaluating response to treatment. Diffusion is a physical process that results from the thermally driven, random motion of water molecules. In tissues that are highly cellular (e.g. tumour tissues), water diffusion is highly restricted because of the tortuosity of the extracellular space and the higher density of hydrophobic cellular membranes. DW-MRI is unique in its ability to provide information that reflects tissue cellularity and the integrity of cellular membranes. Diffusion is mainly of two types: isotropic and anisotropic. Biological tissues mainly exhibit anisotropic pattern of diffusion. In anisotropic materials, a single number cannot describe diffusion, but a [3 × 3] array called the diffusion tensor. The three diagonal elements (Dxx, Dyy, Dzz) of the tensor represent diffusion coefficients measured in the laboratory frame of reference along each of the principal (x-, y- and z-) directions. The calculation of an ADC map requires at least two b-values, and this is often done as a postprocessing step. ADC value is the slope of the line that is superimposed on the plot of the logarithm of relative signal intensity (y-axis) versus b value (x-axis). More accurate ADC values can be obtained by using more DW images with different b values. Final image with different ADC values calculated for each pixel of an image is referred to as an ADC map. ADC values can be ascertained by drawing region of interests (ROIs) on the ADC map. The regions with more restricted diffusion and, therefore, higher DW signal show lower ADC values. “ADC” is used to refer to the mean diffusion in a voxel, sometimes taken as the sum or average value of the tensor’s diagonal elements. DWI pulse sequence framework: Modern DW sequences were originated from the pulsed gradient SE technique developed by Edward Stejskal and John Tanner in mid-1960. Symmetric and strong diffusion-sensitizing gradients (DGs) are applied on either side using 180 degrees pulse. The phases of stationary spins are unaffected by the DG pair since the second gradient lobe reverses the phase accumulated by the first gradient lobe. Diffusion spins tend to lose signal between lobes. An image acquisition module follows the second DG. This is typically an echo-planar sequence using rapidly oscillating phase and frequency gradients that generate multiple gradient echoes. Rapid image acquisition minimizes corruption of DW contrast by vascular pulsation and bulk motion. Other modules (such as fast spin echo [FSE]) are possible too. It is typically performed using a single-shot echo-planar (SS-EPI) sequence with an advantage of speed but suffers from issues such as susceptibility artifacts/distortions, low SNR and spatial blurring. So, to improve image quality (at the expense of increased imaging time), read-out-segmented echo-planar diffusion methods (RS-DWI) have been developed. This method reduces susceptibility artefacts and improves spatial resolution compared with SS-EPI methods by employing fast (turbo) SEs and sample k-space in a radial fashion. Image quality is significantly improved, and the motion artefacts are reduced. These sequences are superior to RS-DWI EPI methods where susceptibility artefacts are most problematic, such as in evaluating temporal bone cholesteatomas. In RS-DWI, k-space sampling occurs in a small number of shots with each shots having limited transversal of k-space in the read-out direction. Acquisition of 2D navigator read-out segments is done during the second and the subsequent echoes since the motion artefacts between segments can create substantial artefacts. At k-space centre, the navigator is repeatedly acquired to account for shot-dependent nonlinear phase differences that arise from nonrigid motion of the head or other imaged body part. However, this technique has some limitations, for instance, the image acquisition time may be comparatively longer when compared with RS-DWI and the imaging artefacts may limit acquisition primarily to the axial plane. Additionally, the use of turbo SEs increases SAR and produces more tissue heating compared with gradient echo EPI methods. Zoom diffusion: This diffusion technique makes use of a noncoplanar excitation, an outer volume suppression and inner volume suppression. RF pulses applied at an angle to each other are referred to as “noncoplanar” pulses. In Zoom DWI, the 180 degrees refocussing pulse is applied oblique to the 90 degrees slice excitation pulse. To minimize artefacts that can occur from fat signal, fat suppression is applied to the inner volume, the reduced FOV. These artefacts include water–fat shift and ghosting artefacts from the patient’s skull. The artefacts reduce the sensitivity of the DW scan and may mimic lesions or obscure lesions. The benefits of Zoom DWI are mainly related to the acquired reduced FOV in phase-encoding direction. The reduced FOV intrinsically enables shorter EPI echo train lengths. Among other things, a small EPI factor helps to reduce artefacts, such as susceptibility and image blurring. Whole-body DWI: Till 2004, the possibility of DWI of the body under free breathing conditions was not possible due to irretrievable loss of DW information as a result of cardiac and respiratory motions. However, Takahara et al. overcame this limitation by developing a technique known as DW whole-body imaging with background body signal suppression (DWIBS), an acronym still used by Philips Healthcare for their product sequence. Whole-body imaging is more reliably performed at 1.5 T because of increased susceptibility artefacts at higher field strengths. Moderately strong diffusion-sensitizing gradients are used (b-values ∼1000 s/mm2), preceded by STIR-like inversion pulse for fat suppression. Blocks of approximately 60 axial images 4- to 5-mm thick are usually acquired using parallel imaging and echo-planar read-out, with imaging times in the range of 7 min per station. The blocks are digitally stitched together, and when displayed in an inverted mode, the image resembles PET-CT. Lymphoma, prostate cancer and small-cell carcinoma have been the most widely studied. Further optimization of DWI sequences and new advancements to enhance the utility of DWI are currently being focussed in research. Non-EPI sequences (turbo-FLASH, HASTE, SSFP) or dual-source parallel RF excitation DWI are novel strategies to overcome the disadvantages of 3 T systems. Periodically rotated overlapping parallel lines with enhanced reconstruction technique based on the FSE sequence has been used for DWI acquisitions to nullify the geometric distortion. To address the motion-related distortions, a new acquisition technique known as “tracking-only navigator echo” is used. ADC histogram: Voxel-based analysis of the apparent diffusion coefficient (ADC) can be performed by histogram analysis. ADC histogram is generated with relative ADC value represented in x-axis and the number of voxels at any relative ADC value represented in the y-axis. Future directions of DWI: For both image acquisition and data analysis across imaging platforms, future standardization of protocols (e.g. type of sequence, number of motion-probing gradient directions, b values, and TRs and TEs) are important. Current commercial software tools available for quantitative analysis are basic and do not allow more complex processing. Some improvements such as noise filtration for noisy DW images and image registration to reduce errors in ADC calculations to improve the quality of the ADC data can be done. Intravoxel incoherent motion (IVIM): When DW-MRI is performed in well-perfused body tissues within the b value ranges usually applied for body imaging (i.e., 0–1000 s/mm2), microcirculation within the normal capillary network along with water diffusion can contribute to the measured signal attenuation (e.g. 0–100 s/mm2). Signal attenuation is caused as a result of phase dispersion at DW-MRI where Le Bihan et al. termed the behaviour of protons that display signal attenuation at DW-MRI as showing IVIM. Microcirculatory perfusion (flow of blood within capillaries) with no specific orientation is a type of pseudodiffusion, depends on the velocity of the blood flow and vascular architecture and is b value dependent. However, because of the larger distances in proton displacement during the application of the motion-probing gradients, the rate of signal attenuation is greater than the tissue diffusion. Therefore, in the normal perfused tissue, pseudodiffusion at higher b values accounts for only a small proportion (if any) of the measured signal in each imaging voxel, unlike lower b values. Using IVIM-based analysis, it is possible to derive quantitative indices describing tissue water diffusivity, tissue perfusion (pseudodiffusion coefficient) and the perfusion fraction of tissues, displayed as parametric maps. IVIM biomarkers: Pseudodiffusion, perfusion fraction and kurtosis provide information about the capillary flow, the rate of flow and extracellular architecture, respectively. D, diffusivity: This implies on how freely a proton can move around in a particular tissue under investigation. Diffusivity is considered as a sign of viability. A dying or necrotic tissue shows a decreased diffusivity in comparison with a corresponding normal tissue. In case of active malignancy, due to the higher mitotic rate, the cells encroach into the extracellular space and hence the diffusivity along the extracellular space. The same applies in the case of extracellular depositions. f, Perfusion fraction: It denotes the percentage of capillaries constituting a voxel. It is a fraction with the lower limit of 0 denoting no capillaries and the highest value of 1 denoting it is 100% capillaries. The perfusion fraction denotes the vascularity of the tissue under investigation. It is in fact showing the size of the vascular compartment. This parameter is very useful in tumour follow-up analysis where a decrease in vascularity posttreatment is considered as a good response to the treatment. D*, pseudodiffusivity: It denotes the diffusivity contributed by random movement of protons in the blood which is flowing in the capillaries. It gives a measure of the blood flow rate in the tissue under investigation. This also denotes the vascularity of a tissue. Applications of IVIM: The signal attenuation with increasing b value conforms to a biexponential behaviour in prostate gland. Both fast (pseudodiffusion) and the slow components (tissue diffusivity) of diffusion were lower in prostate cancer compared with normal peripheral or transitional zones. Perfusion fraction of pancreatic carcinoma was shown to be lower than or having a minor overlap with the normal pancreatic parenchyma. Perfusion fraction can be helpful to identify and characterize pancreatic malignancy. IVIM DW-MRI of intraabdominal organs, such as the kidneys and liver, revealed a biexponential pattern of signal attenuation. The calculated ADC values using all b values of these organs were significantly higher than the corresponding tissue diffusion coefficients. Furthermore, the estimated perfusion fraction was lower in the colorectal liver metastases in comparison with the normal liver parenchyma (in keeping with the hypovascular nature of these lesions). Kurtosis: Alteration of a Gaussian pattern of distribution is known as kurtosis. Diffusion kurtosis imaging (DKI) provides a good model of diffusion and captures the non-Gaussian diffusion behaviour as a reflective marker for tissue heterogeneity. K, kurtosis: Diffusion kurtosis implies the skewness or deviation from the expected diffusion behaviour. When the cells are tightly packed or when there are extracellular depositions, the protons are no freer to diffuse. They bounce back against the cells or collide with the deposition in the extracellular space. As a result, the fall in signal intensity as we go up to the higher b-values is lesser than the expected. The extent of this deviation is what is called the kurtosis. So the more the complexity of cellular architecture, the more malignant the tissue, and the more the value of kurtosis. Kurtosis parameters: Diffusion kurtosis also varies across directions being measured. The directional diffusion profile can be captured using a 3 × 3 tensor matrix with three eigenvectors oriented along the three principal axes of the diffusion ellipsoid and the corresponding eigenvalues representing the diffusion coefficient along the each of the principal axes. Directional kurtosis is a more complex spatial distribution characterized by a 3 × 3 × 3 × 3 tensor matrix. An interpretation of the kurtosis tensor interpretation is yet to be explored. Mean kurtosis (MK), the average of the diffusion kurtosis along all diffusion directions, axial kurtosis (AK), the kurtosis along the axial direction of the diffusion ellipsoid, and radial kurtosis (RK), the kurtosis along the radial direction of the diffusion ellipsoid, are the most commonly used DKI parameters having more direct physical relevance to the diffusion tensor. Applications of kurtosis: Hui et al. found heterogeneous kurtosis imaging characteristics within ischaemic tissue that was not evident with conventional diffusion imaging measuring apparent diffusion coefficient. It was found that there was significantly higher absolute percentage change using kurtosis imaging and specifically that AK has the greatest increase. Due to increased tissue heterogeneity and an osmotic imbalance from enlargement of axons and dendrites during infarction, absolute percentage change using kurtosis imaging was significantly higher, especially axial kurtosis, which has the highest increase. Axial kurtosis (parallel to the direction of the axons) is primarily affected by intracellular structures, whereas radial kurtosis (perpendicular to the direction of the axons) is influenced by cellular membranes and myelin sheaths. The highest change in AK is consistent with the proposed mechanism of axonal beading in ischaemic tissue. Moreover, this supports the theory that changes in kurtosis patterns in ischaemia are primarily due to the intracellular microenvironment. Future directions of IVIM and kurtosis: DW-MRI performed using a range of b values can measure estimates of tissue perfusion and diffusivity based on IVIM analysis. Clinical studies have shown the potential of these parameters for various disease assessments, especially in oncology. Only the fast diffusion component suppressed using low b values is sensitive to microcapillary perfusion. Other bulk flow phenomena such as tubular flow or glandular secretion can contribute to signal attenuation, which is difficult to distinguish from perfusion effects. Approximately 100 billion neurons within the human brain are connected via the white matter axons forming complex networks to enable us to function. Diffusion tensor imaging (DTI), a neuroimaging method, makes it possible to map these axonal networks. DTI is a technique which was first introduced in the year 1994 by Peter Basser, based on principal of diffusion tensors. Given that axons act as conduits of communication between neurons, and have a specific orientation, thickness, path, etc. water molecules within them tend to diffuse more freely along the principal direction of the axon compared with other directions. Because of this directional dependence of water within the cerebral white matter, water diffusivity within the white matter is not uniform in all directions, i.e. diffusivity is anisotropic within the white matter. When measured using diffusion-weighted imaging, typically it is the direction of the maximum diffusivity, along the white matter fibre, is captured within the final image. By connecting the principal diffusion directions from one voxel to the other, the path of the white matter fibres could be traced – giving rise to the capability to map white matter tracts of the brain. Thus, DTI utilizes water diffusion as a probe to determine or map the cerebral white matter networks. DWI is MR sequence which is based on the measurement of thermal Brownian motion of water molecules, termed “diffusion” wherein signals have solely generated the movement of water molecules. For measuring water diffusion using MRI, the signal has to be sensitized to diffusion-based motion of water molecules during acquisition. This could be done using linear field gradients since random motion in the presence of a spatially varying magnetic field leads to irreversible loss of signal coherence, leading loss of signal amplitude, which is proportional to the field gradient applied. In MR image acquisition, linear field gradients are inherent to image acquisition for steps such as slice selection, phase encoding or gradient echo, all of which do lead to slight diffusion weighting of the signal. However, this weighting is quite small to detect water diffusion over small distances. Explicitly applied, large amplitude field gradients are utilized to sensitize the signal to water diffusion over small distances. By applying these large magnetic field gradients, typically in three orthogonal directions, diffusion-related signal changes could be made the dominant source of contrast between different tissues, enabling visualization of diffusional changes and their directional dependence in the images. Diffusion tensor imaging (DTI) is a variant of DWI, where the number of directions along which diffusion sensitizing gradients are applied is usually six or greater. This is to enable extraction of the diffusion tensor parameters, which allow us to evaluate the principal orientation of the diffusion and more. Diffusion weighting could be obtained by inserting diffusion sensitizing gradients prior to the 180 and after the 180 refocussing pulses, within a typical SE imaging acquisition. DW images are heavily T2-weighted images by virtue of their long TE. For the scheme described, for tissues where diffusion is negligible, the diffusion sensitizing gradients do affect the MR signal since the first gradient dephases and the second rephrases it. On the other hand, in the presence of diffusion, since loss of coherence due to diffusion is irreversible, both sensitizing gradients lead to loss in signal amplitude. In tissues like white matter, which are highly ordered and have anisotropic diffusion, however, the diffusion constant parameter cannot be represented using a single quantity. To characterize anisotropic diffusion, it can be modelled using a diffusion tensor. Estimating the diffusion tensor (D), however, requires diffusion weighted measurements in at least six non-colinear directions, along with an image without diffusion weighting (b = 0; b0). Image collected with b = 0 image typically has a heavy T2 weighting without any diffusion weighting. Tensor representation of diffusion is typically done through a 3 × 3 symmetric matrix, with six independent tensor elements, which help in characterizing water diffusion within a region. D ¯ = ( D xx D xy D xz D xy D yy D yz D xz D yz D zz )
1.24: Advancements in MRI
Parallel imaging
Sensitivity encoding/array coil spatial sensitivity encoding
Generalized autocalibrating partial parallel acquisition/autocalibrating reconstruction
Artefacts due to parallel imaging
Simultaneous multislice/multiband sense
Compressed sensing
Compressed sense
Multitransmit technology
Development history
Technical description
Multidrive
Clinical application
Benefits of multitransmit
Digital broadband magnetic resonance imaging
Technical description
Clinical application
Hybrid magnetic resonance imaging
MRgFUS
Positron emission tomography/magnetic resonance imaging
Sequential and simultaneous positron emission tomography imaging
MR-LINAC
Magnetic resonance imaging surgical suite
How MR-OR is performed?
Magnetic resonance radiation therapy
Magnetic resonance elastography
Technical overview of magnetic resonance elastography
Chapter II: Magnetic-resonance-guided biopsy
Diffusion-weighted imaging
Introduction
Apparent diffusion coefficient
Diffusion-weighted imaging read-out mechanisms
Kurtosis biomarkers
Diffusion tensor imaging
Technical description
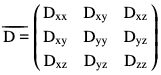
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine