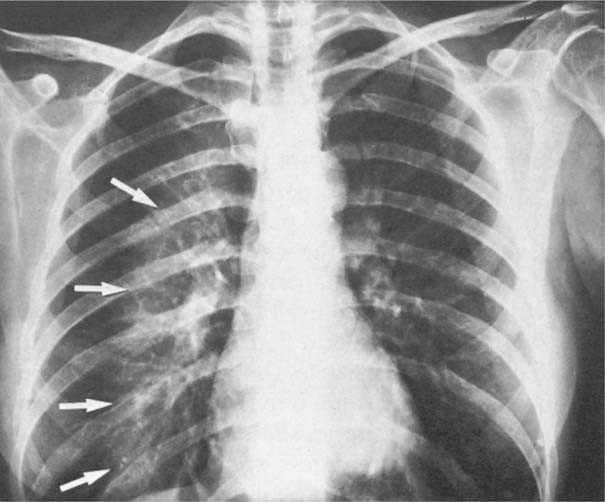
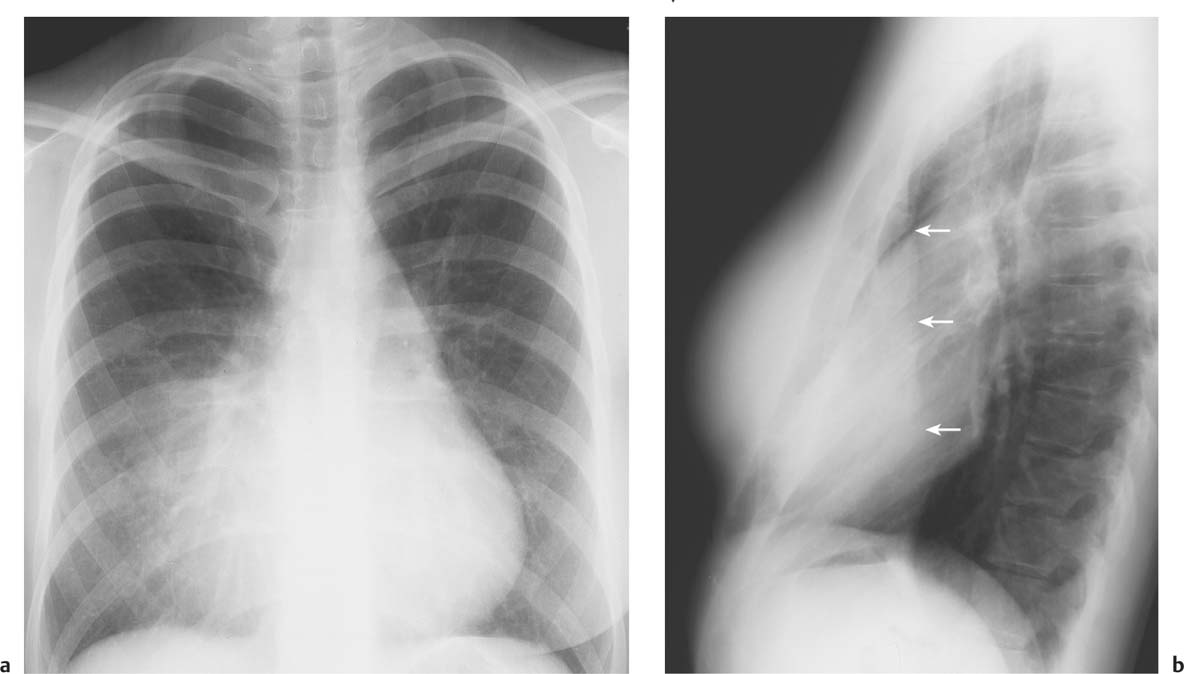
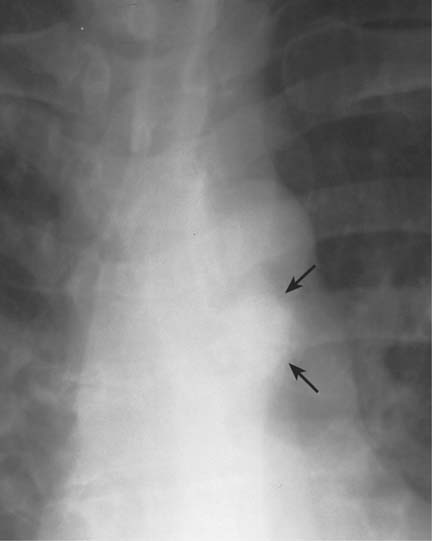
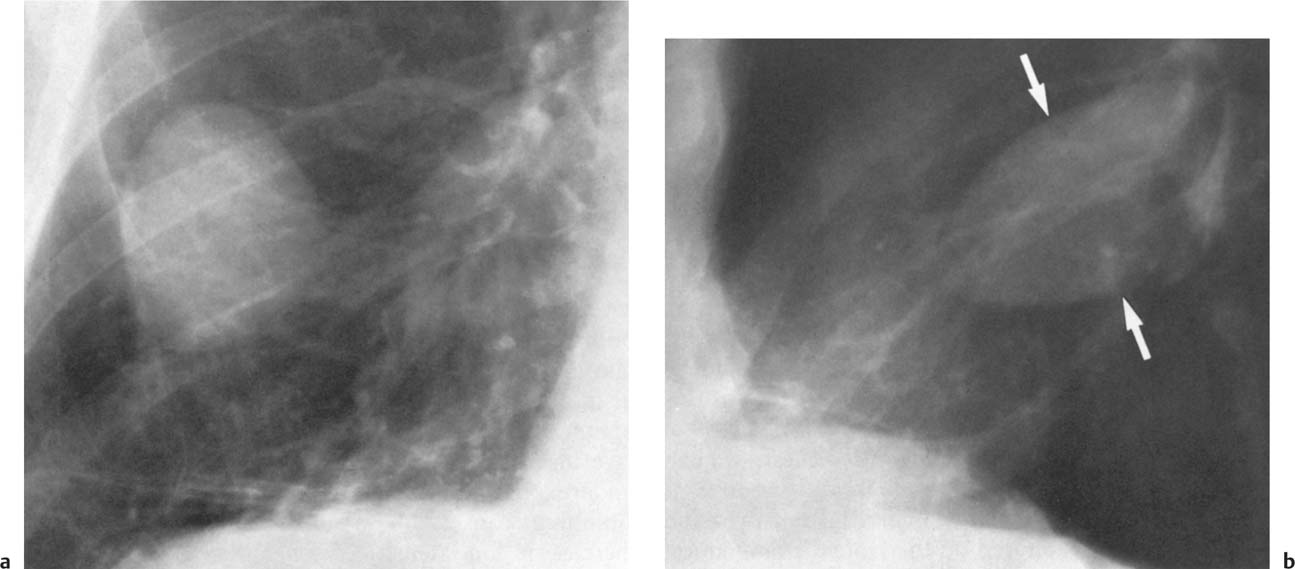
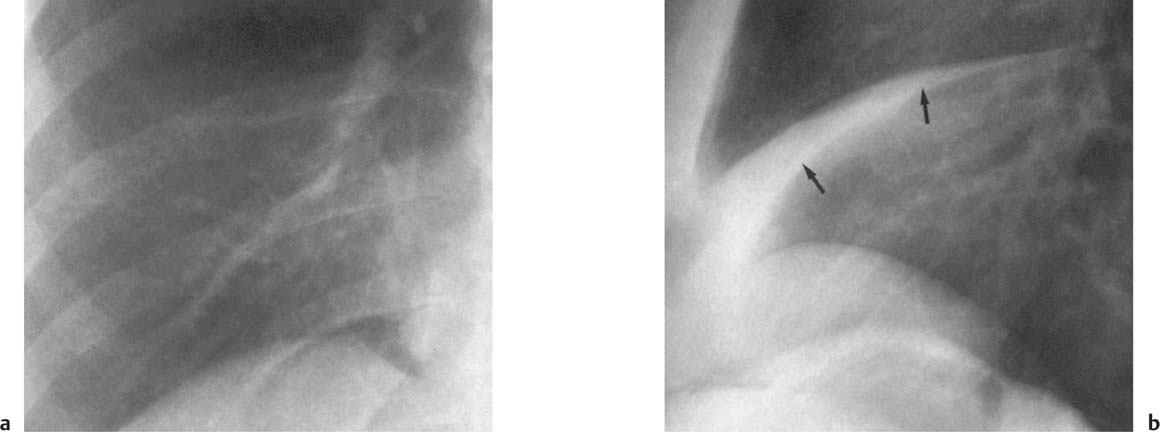
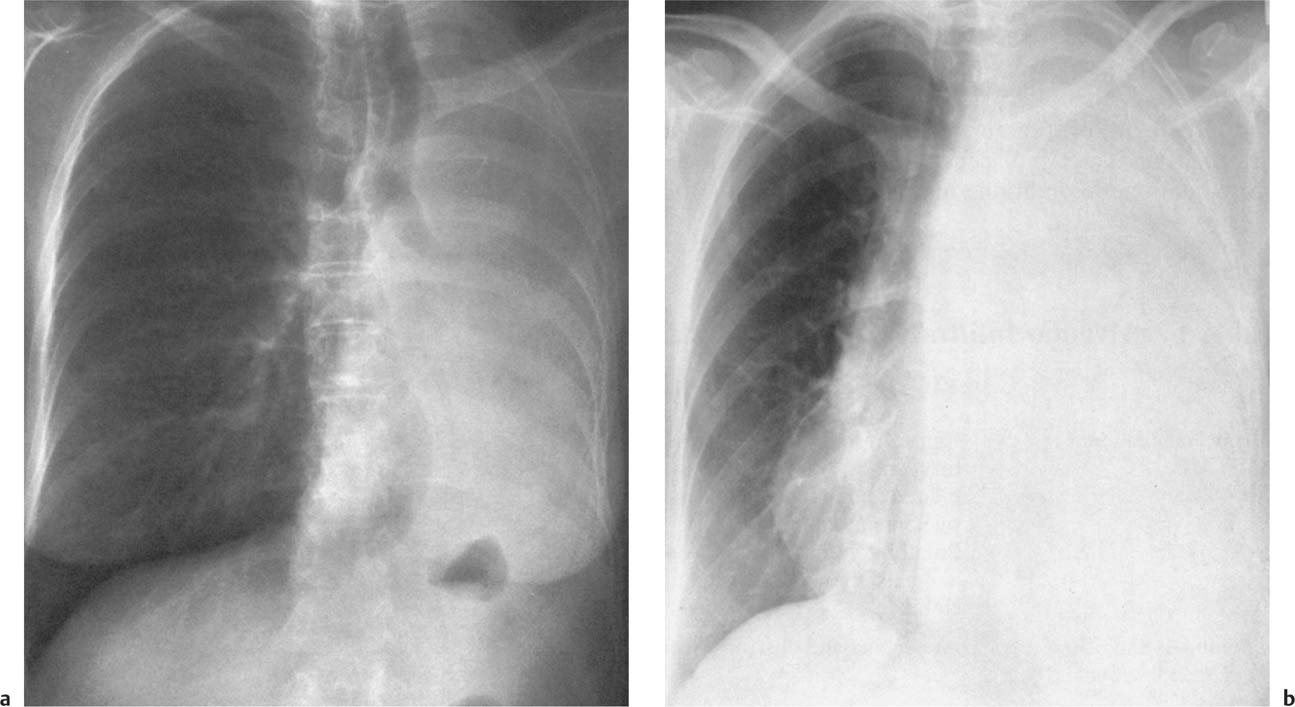
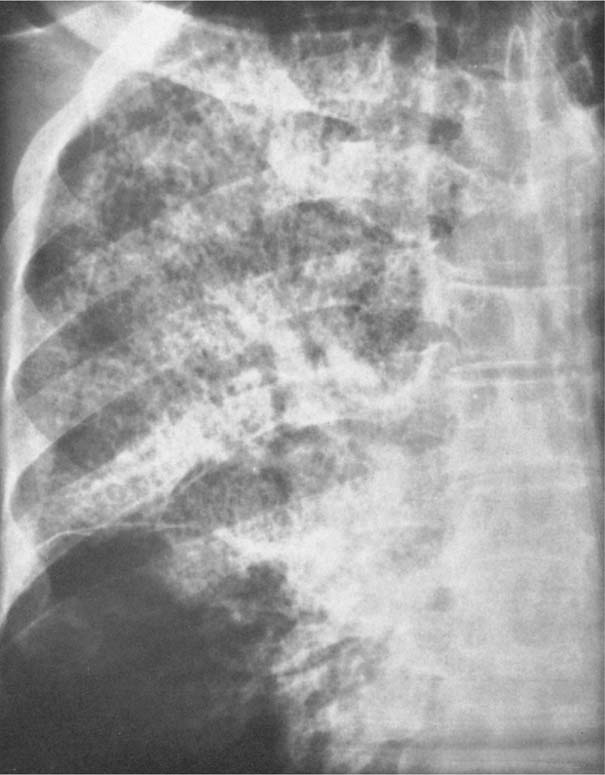
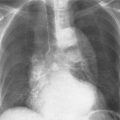
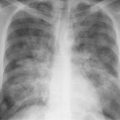
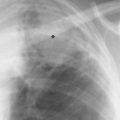
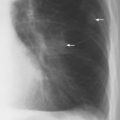
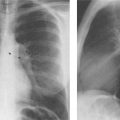
5 Alveolar Infiltrates and Atelectasis An increase in the radiologic density of the lung may be caused by a pulmonary or an extrapulmonary process. Differentiation between these two entities should be attempted first whenever an increased density is observed in the lung (Figs. 5.1–5.3). Normal and pathologic structures of the chest wall can cause an opacification in a lung field and simulate at times pulmonary disease. In women, the density of the lower lung fields is altered on the frontal examination by the presence and size of the breasts. Similarly, the pectoralis muscle, particularly when strongly developed, or an overlying scapula may produce a localized increase in lung density. Whereas the recognition of normal soft tissue and bony structures does not usually cause any difficulty because of their constant anatomic location, a pathologic condition such as a tumor or a hematoma of the chest wall is more likely to be confused with a pulmonary process. Radiographic evaluation in two projections and/or clinical examination of the patient should, however, allow easy differentiation between a chest wall and a pulmonary lesion. A unilateral increase in lung density is found when the frontal chest radiograph is taken in a slightly rotated position. Fig. 5.1 Artifact caused by long hair combed in a ponytail simulating an infiltrate in right lung (arrows). Fig. 5.2a, b Pectus excavatum deformity. A large opacity is evident in the lower portion of the right hemithorax contiguous with the thoracic spine mimicking a right middle lobe infiltrate (a). This is however a normal finding in patients with severe pectus deformity (b) caused by the posteriorly displaced sternum (arrows) resulting in compression of the adjacent right lung parenchyma and displacement of the heart towards the left. Fig. 5.3 Osteophytosis in a costovertebral joint. A round opacity (arrows) is overlying the left seventh rib posteriorly in the area of the costovertebral joint caused by advanced degenerative changes including marked hyperostosis in this articulation that could be mistaken for either a pulmonary or calcified mediastinal lesion. Fig. 5.4 Loculated pleural effusion in major fissure (lower half). a Anteroposterior projection: A homogeneous and well defined density projects into the right lower lung field. Smaller (thinner) fluid accumulations in that location are usually poorly defined and may mimic a right middle lobeatelectasis. b Lateral projection: The encapsulated fluid accumulation is spindle-shaped, with characteristically convex borders (arrows). Its long axis parallels the major fissure, which can often be seen exiting the density at one or both poles. The lung, on which the spine is superimposed because of the rotation, usually demonstrates an increase in density. This is produced by the musculature around the spine that absorbs a significant portion of the roentgen beam passing through. A similar effect may also be found by incorrect centering of the roentgen beam or in patients with scoliosis. It is easy to recognize when the chest radiograph is centered off midline, as there is a variable distance between the lateral chest walls and the film margins: the side more distant from the midline is less exposed and appears lighter. On a supine chest film a pleural effusion layering out posteriorly may also mimic a unilateral underexposed (light) film. Common causes for a bilateral and symmetrical increase in lung density of a healthy person are poor inspiration and underexposure of the film. Pleural abnormalities may occasionally be difficult to differentiate from pulmonary lesions. A loculated effusion in the minor fissure could be mistaken for a neoplasm, although its location and radiographic appearance (a sharply demarcated and spindle-shaped lesion in both frontal and lateral projection) are quite characteristic. Furthermore the extremities of the loculated interlobar effusion blend imperceptibly with the interlobar fissure when viewed tangentially. Loculated fluid accumulation in the lower half of the major fissure is occasionally difficult to differentiate from a right middle lobe atelectasis. The encapsulated fluid has convex borders in the lateral projection and does not obscure the right heart border in the anteroposterior projection (Fig. 5.4). Furthermore, the minor fissure may be visible as a separate entity in one or both projections. Fig. 5.5 Right middle lobe atelectasis. a Anteroposterior projection: A poorly defined pulmonary density obliterates the adjacent right heart border (positive “silhouette sign”; see Fig. 5.8, for negative “silhouette sign” with right lower lobe atelectasis). b Lateral projection: A well defined triangular density with characteristic concave inferior border (arrows) is evident. Furthermore, the minor fissure cannot be seen in either projection as a separate shadow, since this fissure constitutes the superior border of the triangular density. Fig. 5.6 Complete opacification of one hemithorax (atelectasis versus pleural effusion). a Total atelectasis of the left lung caused by bronchogenic carcinoma. Opacification of the left hemithorax is associated with signs of loss of volume: shifting heart and mediastinum toward the atelectasis, approximation of ribs, and elevation of ipsilateral diaphragm. b Large left pleural effusion caused by ovarian carcinoma. Opacification of the left hemithorax is associated with signs of mass effect: shifting of heart and mediastinum away from the pleural effusion and depresston of the ipsilateral diaphragm. An atelectasis involving the entire right middle lobe has straight or concave margins in the lateral projection, and tends to obliterate the adjacent right heart border (positive “silhouette sign”) in the anteroposterior projection (Fig. 5.5). The minor fissure is never seen as a separate linear density. In the upper half of the major fissure, an encapsulated fluid accumulation may, particularly on the frontal view, be confused with a pulmonary mass. On the lateral view, however, the encapsulated fluid has a characteristic elliptical shape with the long axis of the density paralleling the course of the major fissure. The encapsulated fluid blends at both ends with the fissure, provided the latter is visible. An infrapulmonary effusion may atypically extend into the posteromedial gutter, simulating a lower lobe atelectasis. Opacification of a hemithorax by either massive atelectasis or effusion should not be difficult to differentiate, since the former is associated with radiographic signs of loss of volume (e.g., shifting of the heart and mediastinum toward the atelectasis, approximation of ribs, and elevation of the ipsilateral hemidiaphragm), where as the latter is an expanding process displacing the adjacent organs away from the effusion (Fig. 5.6). Complete Opacification of a hemithorax with signs of volume loss similar to an atelectasis is also found after pneumonectomy. Fig. 5.7 Air bronchogram in the right upper lobe. Air containing bronchi are visible, since they are surrounded by an alveolar infiltrate caused by pneumococcal pneumonia. In alveolar lung disease as defined in this chapter, the air in the alveolar and peripheral airways is replaced by fluid or tissue, resulting radiographically in opacities ranging in size from a few millimeters to several centimeters. Alveolar infiltrates may involve a segment or even a whole lobe, in which case boundaries of the resulting parenchymal consolidation are sharply demarcated. On the other hand, a large number of superimposed and partly confluent alveolar infiltrates may produce nonhomogeneous and rather poorly defined lung densities. Air bronchograms are commonly found in alveolar lung disease, where the air-containing bronchi become clearly visible because of the surrounding infiltrates (Fig. 5.7). However, an air bronchogram is not diagnostic of alveolar disease, since an increase in the density of the peribronchial tissue is occasionally caused by interstitial or atelectatic lung disease. Whereas the absence of an air bronchogram in a lung density does not rule out alveolar disease, its presence is diagnostic for lung parenchyma involvement as opposed to pleural or extrapleural disease. The radiographic demonstration of an air bronchogram leads strongly away from a diagnosis of a malignant pulmonary neoplasm, with the exceptions of alveolar cell carcinoma and lymphoma. Air in the lung can be replaced by either fluid, cells, or solid substances under the following conditions: 1 the osmotic pressure of the blood is too low (e.g., hypoproteinemia); 2 the blood pressure in the capillaries is too high (e.g., heart failure); 3 the capillary permeability is increased (e.g., inhalation of noxious gases); 4 the barrier between blood and air space is defective (e.g., bleeding); 5 liquid or solid materials are aspirated; 6 abnormal material is secreted (e.g., cystic fibrosis) or deposited (e.g., alveolar proteinosis); and 7 cells are growing (e.g., neoplasm) or invading (e.g., infection and inflammation) the air space. In an atelectasis the resorbed air is not replaced by any material. All the aforementioned conditions may radiographically produce opacities within the lung parenchyma and their differentiation depends on size, location, and distribution, the association of other radiographic findings, and knowledge of the patient’s clinical history and physical examination. The differential diagnosis of alveolar infiltrates and atelectasis is discussed in Table 5.1.
Disease | Radiographic Appearance | Comments |
Bronchial adenoma (Fig. 5.8) | Centrally located tumors arising from major bronchi (80%) often present as recurrent segmental and lobar atelectasis and/or obstructive pneumonia. Pulmonary, hilar and bony metastases (lytic and blastic) occur occasionally with carcinoids. | 90% in patients between 30 and 50 years old. Slightly more common in females. Histologically 3 types: 1 carcinoid adenomas (90%) including oncocytoid type; 2 cylindromas (7%) and 3 mucoepidermoid tumors (3%). |
Papillomas and other benign endobronchial tumors | Atelectasis and obstructive pneumonia | Rare. Papillomas may originate in larynx or trachea. They may be multiple and commonly occur in children. |
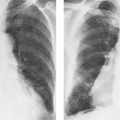
Bronchogenic carcinoma (Fig. 5.9) | Persistent atelectasis and obstructive pneumonia (usually segmental, but may also be lobar or, less commonly, involve a whole lung) is most common radiologic presentation. Ipsilateral hilar enlargment and when further advanced, mediastinal widening is often associated with the parenchymal disease. Pleural effusions are simultaneously present in approximately 10% of all cases. Pancoast or superior pulmonary sulcus tumors present as unilateral apical mass, often with destruction of the adjacent rib. | Approximately 75% occur in males in their 5th and 6th decades. Inhalation of carcinogens (cigarette smoke, asbestos, etc.) are predisposing factors. Incidence in cigarette smokers ten times higher than in nonsmokers. Carcinomas also originate in tuberculous scars with higher than purely coincidental incidence. Histologically adenocarcinomas (50%), squamous cell carcinomas (30%), small cell carcinomas (15%) and large cell carcinomas (5%) are distinguished. Pancoast tumors often present clinically with Horner’s syndrome. |
Bronchioloalveolar carcinoma (alveolar cell carcinoma)(Fig. 5.10) | Peripheral homogeneous consolidation ranging in size from 1 cm in diameter to the involvement of an entire lobe. Air bronchograms are often present. Diffuse bilateral involvement with fluffy confluent infiltrates occurs in a more advanced stage. Hilar enlargement and pleural effusions are occasionally associated with the pulmonary involvement. | Most commonly found in middle-aged patients, without sex predilection. |
Lymphoma (Fig. 5.11) | Perihilar infiltrate is the most common parenchymal manifestation. Peripheral consolidations and atelectasis secondary to endobronchial lymphoma are less common. Air bronchograms are often encountered. Hilar and/or mediastinal involvement is usually present with lung parenchyma involvement. | Primary pulmonary lymphoma is rare and radiographically indistinguishable from pseudolymphoma, a rare benign condition not associated with hilar or mediastinal adenopathy. Any alveolar lung infiltrate in a patient with known lymphoma is more likely to represent an infectious than a lymphomatous process. |
Kaposi’s sarcoma (Fig. 5.12) | Bilateral, symmetric, poorly defined nodular opacities are characteristic. Nodules measure up to 3 cm and tend to coalesce. Pleural effusions (50%) and hilar or mediastinal adenopathy (10%) may be associated. | Occurs almost exclusively in HIV-infected male homosexuals and rarely in other HIV-infected patients. |
Amyloidosis (tracheo-bronchial form) | Endobronchial amyloid deposition may cause atelectasis and obstructive pneumonia involving a segment to an entire lung. | Pulmonary manifestations occur in primary amyloidosis or in conjunction with multiple myeloma. |
Arteriovenous malformation | Homogeneous, sharply defined and often somewhat lobulated density with lower lobe predilection. Identification of feeding and draining vessel not always possible. Multiple lesions in approximately one-third of patients. | Approximately half of the patients have arteriovenous fistulas elsewhere (hereditary hemorrhagic telangiectasia or Rendu–Qsler–Weber’s disease). |
Pneumonia, bacterial Staphylococcus aureus (Fig. 5.13) | Patchy consolidations without air bronchograms characteristic. Bilateral in over 60%. Both pneumatocele and abscess formations occur frequently and may contain air-fluid levels. Pneumatoceles have characteristically thin walls that differentiate them from abscesses. Pleural effusion (or empyema) is found in 50% of adults and is even more common in children. | Common in hospital patients and compromised hosts. Pneumatoceles are cyst-like lesions resulting from a check-valve-obstructed communication with a bronchus. They are particularly common in children. |
Streptococcus pyogenes | Similar to staphylococcal pneumonia: patchy or homogeneous consolidations without air bronchograms and a high incidence of accompanying pleural effusion. Differentiating features are a lower tendency to form abscesses and pneumatoceles. | Streptococcal pneumonias are often found in mixed or secondary infections. |
Pneumococcus (Streptococcus pneumoniae) (Fig. 5.14) | Homogeneous consolidation with air bronchograms characteristic. Usually confined to one lobe. Another presentation observed with apparently increasing frequency consists of patchy bronchopneumonia-like infiltrates that may be bilateral. Small pleural reactions occur in 20% of cases. Cavitation is rare. | Common in alcoholics and compromised hosts, but also occurs in otherwise healthy people. In children, the disease may present as a spherical, well-circumscribed pulmonary density. |
Klebsiella (Fig. 5.15) | Large well-defined homogeneous consolidation with air bronchograms similar to pneumococcal pneumonia. Differentiating features from the latter are: 1 upper lobe predilection, 2 tendency to expand involved lobe, 3 abscess formation and pleural effusion common. | Common in alcoholics and in elderly patients with chronic pulmonary disease. |
Legionella (Fig. 5.16) | Poorly defined round or diffuse infiltrates central or peripheral in location, usually beginning in one lobe and spreading in two-thirds of cases to the other lung. Pleural effusion may be present. Cavitation and hilar adenopathy does not occur. | Legionnaires’ disease is an acute Gram-negative bacterial pneumonia found in local outbreaks or as sporadic cases. Clearing of pneumonic infiltrates within one month. |
Pseudomonas (Fig. 5.17) | Bilateral patchy infiltrates with predilection for the lower lobes, progressing rapidly to extensive homogeneous consolidations with air bronchograms, are characteristic. Abscess formation is common. Small but radiographically not very conspicuous effusions are usually present. | Almost invariably in compromised hosts who acquire the disease in the hospital. Organism resistant to almost all antibiotics. |
Haemophilus influenzae (Fig. 5.18) | Patchy, poorly defined infiltrates, predominantly in the lower lobes, unilateral or bilateral. Pleural effusions occur frequently and may be the dominant feature, especially in children. | Most common organism cultured from purulent expectorations of patients with chronic pulmonary disease, although its pathogenicity is still in doubt, since it is also commonly found in the flora of healthy people. |
Bordetella pertussis |