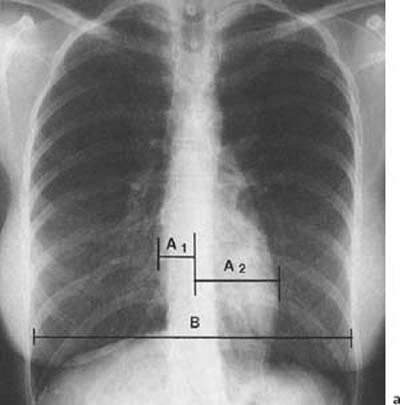
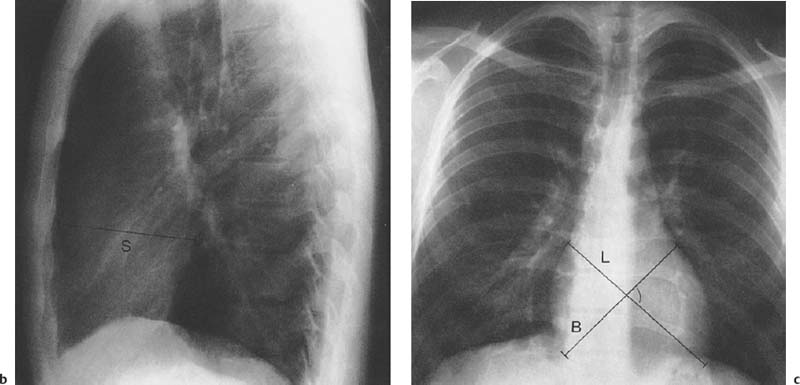
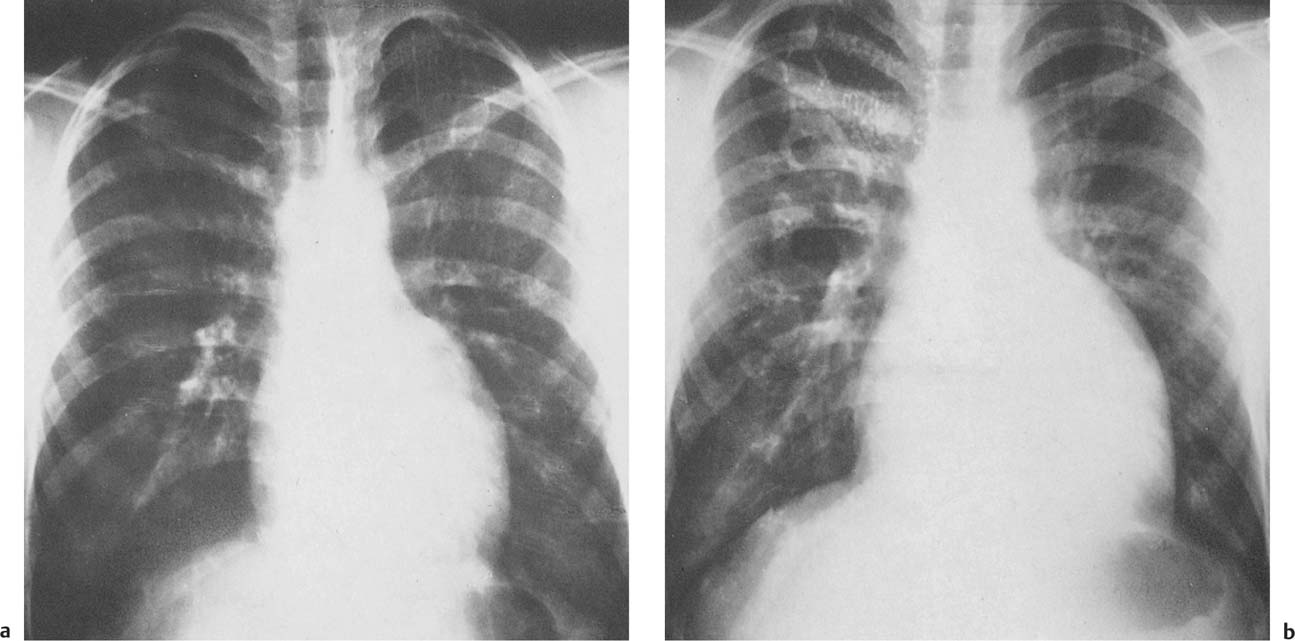
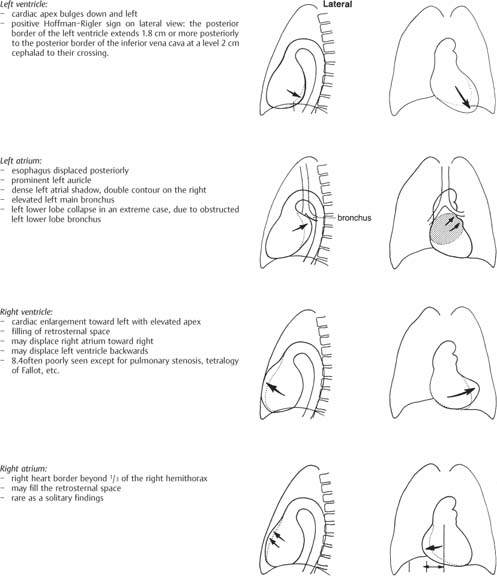
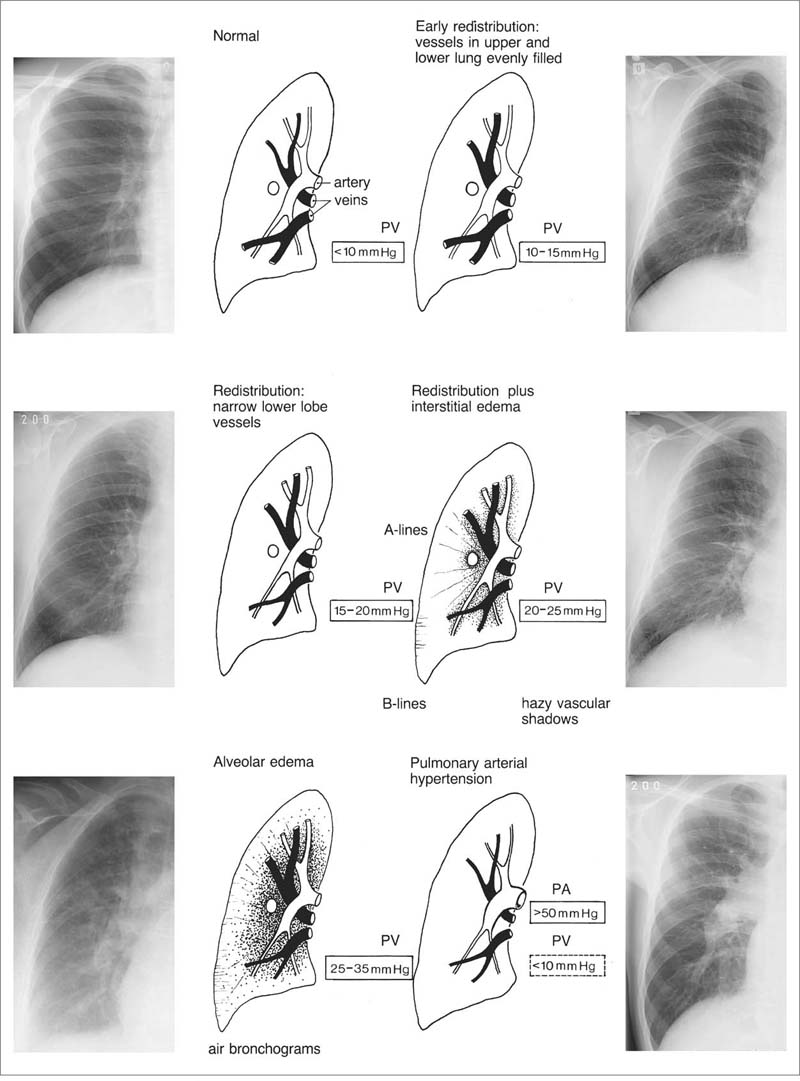
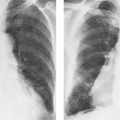
1 Cardiac Enlargement Most of the diseases involving the heart will cause either generalized or localized enlargement of the heart or great vessels. A small heart is usually constitutional (asthenia, senility, wasting) and only rarely reflects a disease (Fig. 1.1). Exceptions to this are adrenal insufficiency, constrictive pericarditis, dehydration, oran asthmatic paroxysm with emphysema, where a relatively small heart may be considered to reflect the effects of the disease process itself. The size of the heart can be estimated in several ways. A simple approach is the cardiothoracic index: the ratio between the total transverse diameter of the cardiac shadow and the internal diameter of the chest (Fig. 1.1a). After the age of 5 years, this ratio normally varies, between 0.4 and 0.5. In smaller children and neonates, the ratio may be as high as 0.6, and during the second month after birth even 0.65. The cardiac volume can be estimated with a fair degree of accuracy by measuring the relative volume of the heart according to the following equation: where L = longest diameter of the heart in posteroanterior view; B = broad diameter of the heart (perpendicular to L); and S = longest sagittal diameter of the heart in lateral view (Fig. 1.1b, c). Factor 0.44 applies to the film-focus distance of 2 m. If this distance is 1.5 m, a factor of 0.42 should be used instead. The surface area of the body is available from charts if the height and weight of the body are known (Fig. 1.2). The relative volume of the heart shows great interindividual variation. Values between 350 cc/m2 and 500 cc/m2 are considered normal for men. In women they tend to be slightly smaller. A single measurement of the relative volume has therefore little diagnostic value, but it may be useful in the follow-up of an individual patient, since many interindividual variables are then excluded. Interindividual variation of interthoracic pressure and other factors still have a great effect (Fig. 1.3). Enlargement of individual chambers of the heart may cause typical configurations, as seen in Fig. 1.4. When there is enlargement of several chambers, the pattern is more complicated and its interpretation may be difficult. In addition to evaluating the configuration of the margins of the heart itself, one has to observe carefully the location and relative size of the pulmonary trunk and aorta as well as of the pulmonary vasculature. The effect of abnormalities of the thoracic cage has to be taken into account as well. The heart may appear enlarged in the frontal projection if the sagittal diameter of the thoracic cage is decreased. This is the case in the straight back syndrome and in pectus excavatum. Fig. 1.1a Small heart in an asthenic woman, age 20. The method of measuring the cardiothoracic index is shown. Fig. 1.1b, c Fig. 1.2 Nomogram for the determination of body surface area of adults (adapted from Documenta Geigy, Scientific Tables, Basel). Join patients weight and weight with a line. In the mid column you find the body surface. Fig. 1.3 Great change in the appearance of heart size is produced by the Valsalva effect. The two exposures were taken a few minutes apart. Fig. 1.4 Typical manifestations of the enlargement of individual cardiac chambers 1 cardiac enlargement – which chambers are enlarged? 2 the size of the aorta- small, wide, narrow, dilated? 3 the vascular pedicle – narrow or wide? 4 right-sided aortic arch? 5 a notch in the aortic arch, resulting in a number 3 appearance? 6 pulmonary vascularity: are the main trunks of the pulmonary artery wide or unusually narrow; is there pulmonary oligemia or hyperemia? 7 the pulmonary artery segment in the left cardiac shadow: large and convex or small and concave. 8 rib notching (Fig. 1.39). The following clinical information is important in evaluating suspected congenital heart disease: 1 is the patient cyanotic or not? 2 Is there right- or left-sided dominance in electrocardiography? 3 Are there typical murmurs? By using the radiographic and clinical information, it is possible to classify congenital heart diseases of childhood into various groups as presented in Table 1.1 or often to predict the correct diagnosis, unless multiple defects exist. In a neonate, only two congenital heart diseases can be diagnosed with great certainty because of their characteristic appearance in chest roentgenogram, namely, total anomalous pulmonary venous return (snowman heart) (Fig. 1.47) and pulmonary stenosis with atrial septal defect (prominent main pulmonary trunk with oligemic lungs). In other cases further clinical information is very helpful in narrowing down the differential diagnostic possibilities. Nowadays ultrasound and Doppler ultrasound examinations have largely replaced plain radiographic analysis of heart. Based on the time of appearance of cardiac failure and cyanosis, the patients can be divided into the following groups: 1 Cardiac failure manifests early (at birth or during the first week) but there is no cyanosis: hypoplasia of the left heart; atresia of the aortic (and possibly mitral) valve; coarctation of aorta. 2 Cardiac failure develops later: ventricular septal defect; patent ductus arteriosus; truncus arteriosus; total anomalous pulmonary venous return (TAPVR). 3 Cyanosis develops at birth or within a week: transposition of great vessels; hypoplastic right heart; Ebstein’s anomaly; tricuspid atresia; obstructive type of TAPVR. 4 Cyanosis develops later during childhood: tetralogy of Fallot; pulmonary stenosis with atrial septal defect. Table 1.3 gives the approximate relative frequency of the congenital heart diseases. In Table 1.5, the diseases are grouped according to their dominant, most common or earliest findings. The differential diagnostic groupings in Table 1.2 may be helpful in narrowing the differential diagnosis in congenital heart diseases in children. In the adult patient with suspected congenital heart disease, the following features are of particular importance: the position of the diaphragm (high or low); degree of inspiration; calcifications; Kerley B-lines; the appearance of the branches of the pulmonary artery; possible arteriovenous malformations in the lung parenchym anomalous veins across the lung field. When blood circulation through the lungs is increased, both arteries and veins are full of blood. This occurs in left-to-right shunting (Table 1.1) and to a milder degree in hyperkinetic conditions (hypervolemia, anemia, polycytemia, pregnancy, hyperthyroidism). A clear oligemia may be a result of a right-to-left shunt or narrow or obliterated pulmonary arterial channels (narrow pulmonary artery, thromboembolic disease or emphysema). Pulmonary blood flow can increase two to three times before the arterial pressure increases. When the precapillary pressure increases, the peripheral branches of the pulmonary arteries appear narrow, but the central trunks are wide. An increase in the pulmonary venous pressure causes characteristic changes in the distribution of blood in the lungs. On upright films of an adult untreated patient, such changes relatively accurately reflect the pulmonary venous and left atrial pressure as diagrammatically presented in Fig. 1.5. These changes, together with the less sensitive changes in the size and configuration of the heart, are the cornerstone of the radiographic diagnosis of congestive heart failure. The findings in the most common conditions involving left ventricular strain are presented in Table 1.4. Although the radiologic evaluation of the pulmonary venous pressure is relatively accurate, the evaluation of venous pressure of the right side is difficult on roentgenograms and is better assessed from the filling of the veins of the patient’s neck. Occasionally a prominent azygos vein may be seen (Fig. 1.6a). In conditions such as acute myocardial infarction, even the central venous pressure readings do not reliably reflect the patient’s hemodynamic condition. Pericardial effusion mimics generalized cardiomegaly. Although it sometimes may cause a characteristic (bottle-like) configuration of the heart shadow, most of the cases are difficult to diagnose on plain films, and as much as 200 ml of pericardial fluid usually goes undetected in the primary reading. Ultrasound examination CT or MRI are far more sensitive than plain films in detecting pericardial effusion. Fig. 1.5 Manifestations of increased pulmonary venous (PV) and arterial (PA) pressure.
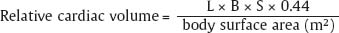

Evaluation of Blood Flow and Blood Pressure in Lungs
Pulmonary vasculature | Cyanosis | Diseases |
Increased | Cyanotic | Mixed lesions with major malformations: Complete transposition Truncus arteriosus (Type I) Single ventricle Common atrioventricular canal Total anomalous pulmonary venous return |
| Acyanotic | Left to right shunts: Atrial septal defect Ventricular septal defect Patent ductus arteriosus Aorticopulmonary defect Partial anomalous pulmonary venous return |
Normal | Acyanotic | Coarctation of aorta Aortic stenosis Subaortic stenosis Pulmonary arterial stenosis Congenital mitral stenosis Endocardial fibroelastosis |
Decreased | Cyanotic | Tetralogy of Fallot Trilogy of Fallot Truncus (pseudotruncus) arteriosus Tricuspid atresia Ebstein’s anomaly Pulmonary stenosis with ASD or transposition |
Unusually small ascending aorta and aortic arch | Atrial septal defect Ventricular septal defect Uncorrected transposition of great vessels |
Enlarged ascending aorta and aortic arch | Patent ductus arteriosus Valvular congenital aortic stenosis Coarctation of aorta Truncus arteriosus |
Concave pulmonary artery segmented in posteroanterior view | Tetralogy of Fallot Tricuspid atresia Ebstein’s anomaly Pseudotruncus arteriosus Uncorrected transposition of great vessels |
Prominent pulmonary artery segment (a moderate prominence is normal under age 20 or in pregnancy) | Patent ductus arteriosus Atrial septal defect Ventricular septal defect Eisenmenger’s complex Valvular pulmonary stenosis (poststenotic dilatation) Cor pulmonale |
Enlarged left atrium in a child | Patent ductus arteriosus Ventricular septal defect Congenital mitral stenosis |
Common | ||
Ventricular septal defect | 20–25% | |
Patent ductus arteriosus | 12–15% | |
Tetralogy of Fallot | 11–15% | |
Pulmonary stenosis without ventricular septal defect | 10–15% | |
Atrial septal defect | 7–14% | |
Transposition of great vessels | 5–9% | |
Coarctation of aorta | 5–9% | |
Congenital aortic stenosis | 3–6% | |
Rare | ||
Tricuspid atresia | 1.2–3% | |
Truncus arteriosus | 1–3% | |
Single ventricle | 2–3% | |
Corrected transposition | 1.2–3% | |
Common atrioventricular canal | 2% | |
Total anomalous venous return | 2% | |
Aortic atresia | 2% | |
Pulmonary atresia | 1–;1.7% | |
Endocardial fibroelastosis | 1% | |
Ebstein’s anomaly | 1% | |
The rest are very rare (less than 1%). | ||
Pulmonary Veins | Size of the Left Ventricle | |
Normal | Enlarged | |
Normal | No heart disease Aortic valve stenosis Arterial hypertension | Athlete’s heart Aortic regurgitation Myocardial damage |
Venous congestion | Acute myocardial infarction Mitral stenosis Hypervolemia Constrictive pericarditis | Congestive heart failure Mitral regurgitation |
Both arteries and veins distended | Atrial septal defect Patent ductus arteriosus Ventricular septal defect Arteriovenous malformation | Possible enlargement of the left ventricle depends on the magnitude of shunting. |
Disease | Radiographic Findings | Comments |
Athlete’s heart (no disease) | Generalized cardiomegaly with left ventricular prominence, no pulmonary vascular changes. | Associated with bradycardia (large stroke volume); secondary to excessive training. |
Arteriosclerotic heart disease (myocardial ischemia) | Chest roentgenogram is usually unrevealing. Variable degrees of generalized or left ventricular enlargement may occur. | Calcification of the coronary arteries indicates coronary atherosclerosis. |
Chest roentgenogram is often unrevealing. Some cardiac dilatation, with variable degrees of pulmonary venous congestion, occurs frequently. Acute pulmonary edema may ensue. | Myocardial aneurysm may develop early in the infarcted muscle or later in the scar. It is seen as a bulge or an unusual prominence in the left ventricular border, which occasionally calcifies. | |
Postmyocardial infarction syndrome (Dressler’s syndrome) | Rapid increase in the heart size due to pericardial effusion, always accompanied by either pleural effusion and/or pulmonary infiltrates in the left base or bilaterally. | Can occur a few days or up to two months following an acute infarction. Left-sided or bilateral pleural effusion is the most common finding (80%) and may occur alone. Pericardial effusion is present in 70% and pulmonary infiltrates in 60% of cases. Response to steroid therapy is striking. |
Congestive myocardial failure |
|
|
Left-sided failure (Fig. 1.9) | Cardiac enlargement, related to the severity of failure and the precongestive heart size. The increase of pulmonary venous pressure can be evaluated as presented in Fig. 1.5). Pleural effusions (bilateral or right-sided). | Pulmonary signs of congestive failure without significant cardiac enlargement. Pericardial calcification is seen in 50%. Often the cardiac configuration resembles that of mitral stenosis. Unilateral left-sided effusion is likely due to causes other than congestive failure. |
Right-sided failure (Fig. 1.10) | Dilatation of the right ventricle and atrium. Widening of the superior vena cava and azygos vein. Liver enlargement. | If right heart failure develops following left heart failure, pulmonary venous congestion from left-sided failure diminishes. |
Chronic arterial hypertension (hypertensive heart) | Left ventricular hypertrophy may produce no radiographic changes or some rounding of the left margin. Left ventricular dilatation causes typical signs of left ventricular enlargement. The aortic knob enlarges concomitantly. Congestive failure may supervene. | Hypertension is most often essential but may be associated with renal or renovascular disease, coarctation of the aorta, adrenal diseases with adrenal hyperfunction, collagen diseases (lupus erythematosus, polyarteritis nodosa), or hyperthyroidism. |
Aortic valve insufficiency (Aortic regurgitation) (Fig. 1.11) | Dilated left ventricle, concave left cardiac border, some dilatation of the ascending aorta and sometimes calcification of the aortic annulus or even the valves. Congestive failure may supervene in advanced cases. Radiographic findings may be subtle although findings in ultrasonography may be obvious. | Insufficiency of the aortic valve can be secondary to: 1 primary damage of the valve (e.g., rheumatoid endocarditis, bacterial endocarditis, congenital valvular deformity, lues, ankylosing spondylitis and Reiter’s disease, mucopolysaccharidosis, spontaneous or traumatic rupture of the valve, or degenerative phenomena); 2 diseases of the aortic wall or annulus fibrosus (e.g., cystic medial necrosis, Marfan’s syndrome, dissecting aneurysm, lues, arterial hypertension); or 3 congenital malformation of the aortic root (e.g., aneurysm of the sinus of Valsalva, congenital coronary fistula, or high ventricular septal defect with prolapse of the noncoronary cusp). Congenital aortic insufficiency is usually due to a bicuspid valve. Acute aortic insufficiency occurs in bacterial endocarditis, rupture of the valve or dissecting aneurysm. |
Combined aortic valve insufficiency and stenosis (Fig. 1.12) | Dilatation of the left ventricle and ascending aorta is more prominent and left atrium may enlarge. May mimic pure aortic stenosis. | Rheumatoid heart disease usually causes a combination of aortic valve stenosis and insufficiency. |
Subvalvular aortic stenosis (idiopathic hypertrophic subaortic stenosis) | Enlarged left ventricle, often enlarged left atrium. Poststenotic aortic dilatation is absent. | Narrowing of the left ventricular outflow tract by muscular hypertrophy. Essentially ultrasonographic diagnosis. |
Supravalvular aortic stenosis | The heart may be enlarged or normal, the aorta is normal or small. | Congenital fibrous ring or thickening above the sinuses of Valsalva. Frequently associated with idiopathic hypercalcemia of infancy. |
Aortic stenosis |
| See Table 1.9 |
High-output heart | Cardiac enlargement, dilatation of the main pulmonary artery, prominent pulmonary vasculature (both arteries and veins). | Associated conditions: Pregnancy or athletic (physiological). Severe anemia including sickle-cell anemia, leukemia, or primary polycythemia. Beriberi (vitamin B deficiency). Hypervolemia (fluid overload). Extrapulmonary arteriovenous fistula. Mild forms occur in extreme obesity, thyrotoxicosis, and pyrexia. Advanced Paget’s disease is a rare cause of high-output heart. |
Myocardiopathy (Figs. 1.13 – 1.14) | Diffuse enlargement of the heart, predominantly of the left ventricle. The aorta appears small as compared with the size of the heart. Pulmonary venous congestion may develop. Moderate left atrial enlargement and fullness of the main pulmonary artery occurs. Superimposed pericardial effusion may further enlarge the cardiac shadow. | The many causes of myocardiopathy may be classified into five groups: 1 Idiopathic, unassociated with extracardiac disease: non-specific myocardosis; endocardial fibroelastosis (neonate or child); postpartum cardiopathy. 2 Infectious: Coxsackie B and other viral infections; Chagas’ disease (South American trypanosomiasis); rheumatic, septic, diphtheric, toxoplasmic. Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|