13 Anatomy of the lower-limb venous system and assessment of venous insufficiency
Venous disorders and treatment
Practical considerations for duplex scanning of varicose veins
Augmentation maneuvers and venous reflux
Grading of superficial and deep venous reflux
Scanning protocol for the lower-limb venous system
Investigation of recurrent varicose veins
Assessment of patients with skin changes and venous ulceration
Endovenous ablation of varicose veins
Other disorders of the venous system
INTRODUCTION
Venous disorders are a common problem and consume a significant proportion of the resources available to health care systems. A review by Callam (1994) indicated that approximately 20–25% of women and 10–15% of men have visible varicose veins. In contrast, results from the Edinburgh Vein Study found that chronic venous insufficiency and mild varicose veins were more common in men (Evans et al. 1999). Significant venous disease can ultimately lead to venous ulceration, resulting in a marked loss of quality of life. Duplex scanning has had a dramatic impact on the noninvasive assessment of the venous system, and it is now the most commonly performed procedure for the detailed investigation of lower-limb venous insufficiency. Lower-limb venous duplex imaging can be used for the assessment of patients with primary or recurrent varicose veins or for the investigation of patients with skin changes and venous ulceration. Additionally, ultrasound is used to guide endovenous procedures, such as foam sclerosant injection, endovenous laser therapy (EVLT), or radiofrequency ablation (RFA), for the treatment of superficial venous disease. This has had a dramatic impact, as this treatment can be performed under local anesthetic as an office procedure. In comparison with arterial duplex scanning, venous duplex investigations can be technically challenging due to the wide range of anatomical variations in the venous system. This chapter covers the basic anatomy of the venous system and scanning techniques used for the assessment of lower-limb venous insufficiency and also includes a description of ultrasound-guided endovenous procedures.
ANATOMY AND PHYSIOLOGY
In this edition we are using anatomical terminology recommended by the Union Internationale de Phlébologie (UIP) published in a consensus document covering duplex assessment of chronic venous disease of the lower limbs and anatomy (Cavezzi et al. 2006). The description of the anatomy below is not exhaustive and the reader is advised to consult the consensus document.
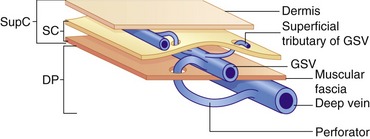
The lower-limb venous system is divided into the deep and superficial veins. The deep veins lie below the muscular fascia. The superficial veins lie between the muscular fascia and the dermis (Caggiati et al. 2002) (Fig. 13.1). There are numerous interconnections between the deep and superficial veins via perforating veins.
Deep venous system
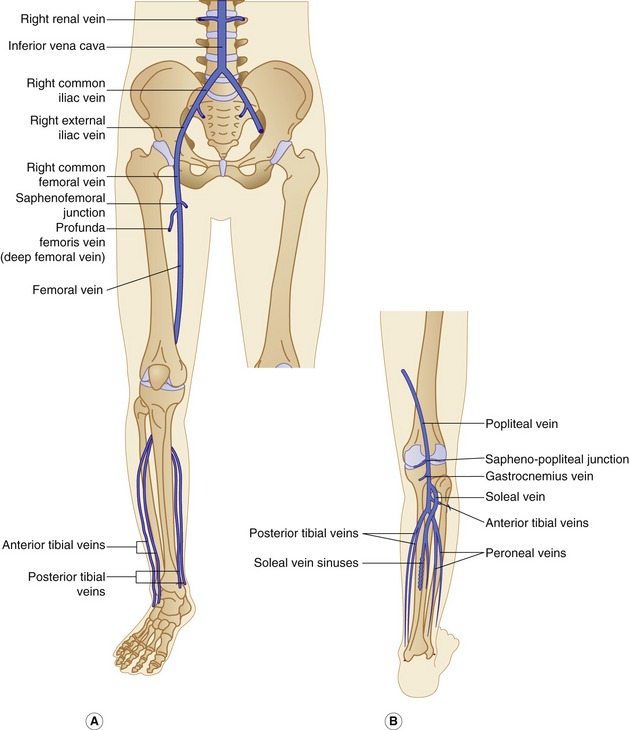
The anatomy of the deep veins is shown in Figure 13.2. Generally, the deep veins are larger than their corresponding artery. The main deep veins of the thigh and calf are the following:
• Deep femoral vein (also called the profunda femoris vein)
• Femoral vein (also called the superficial femoral vein)
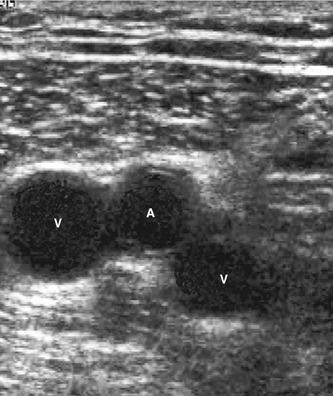
The posterior tibial and peroneal veins are usually paired and are associated with their respective arteries. The paired veins join into common trunks in the upper calf before forming the below-knee popliteal vein. The soleal veins are deep venous sinuses and veins of the soleus muscle that drain into the popliteal vein. They are an important part of the calf muscle pump mechanism (see Ch. 5). The gastrocnemius veins drain the medial and lateral gastrocnemius muscles. The gastrocnemius veins normally drain into the popliteal vein through single or multiple trunks below the level of the saphenopopliteal junction. The anterior tibial vein is paired and associated with the anterior tibial artery and drains to the popliteal vein. The above-knee popliteal vein runs through the adductor canal and becomes the femoral vein in the lower medial aspect of the thigh. The femoral vein runs toward the groin, where it is joined by the deep femoral vein to form the common femoral vein. This confluence is distal to the level of the saphenofemoral junction (SFJ) and common femoral artery bifurcation (see Fig. 9.8). The common femoral vein lies medial to the artery, becoming the external iliac vein above the inguinal ligament (Fig. 13.2). The external iliac vein runs deep and is joined by the internal iliac vein, which drains blood from the pelvis, forming the common iliac vein. The left common iliac vein runs deep to the right common iliac artery to drain into the vena cava, which lies to the right of the aorta.
Superficial venous system
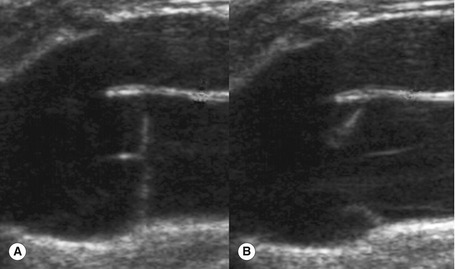
The main superficial veins are the great saphenous vein (GSV) and small saphenous vein (SSV) (Fig. 13.3) (often referred to as the long saphenous vein [LSV] and short saphenous vein, respectively). The GSV and SSV are contained in a separate saphenous compartment, bounded superficially by the hyperechoic saphenous fascia and deeply by the muscular fascia (Fig. 13.4). Branches, tributaries, and cross-communicating veins lie external to the saphenous compartment (Caggiati et al. 2002) (Fig. 13.1). The saphenous compartment resembles the shape of an Egyptian eye when imaged.
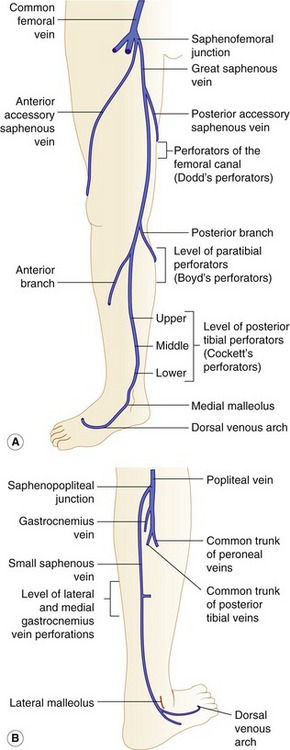
Figure 13.3 Anatomy of the superficial veins, including the position of commonly located perforators. (A) The great saphenous vein. Posterior tibial perforators (sometimes referred to as Cockett’s perforators) are located at distances of approximately 6, 13, and 18 cm above the medial malleolus. Paratibial or Boyd perforators are located in the upper calf, approximately 10 cm below the knee joint. (B) The small saphenous vein.
The GSV and saphenofemoral junction
Tributaries and bifid veins
• The anatomically observed image of the main trunk of the great saphenous vein and a large tributary, lying superficial to the saphenous compartment, does not constitute a true bifid or paired system. The term bifid is properly used when both veins are contained in the same saphenous compartment.
• Bifid and duplicated systems are clinically important as either or both veins may be incompetent and either or both may require treatment. They are also clinically important elsewhere, an example being the femoral vein.
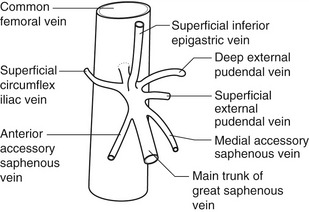
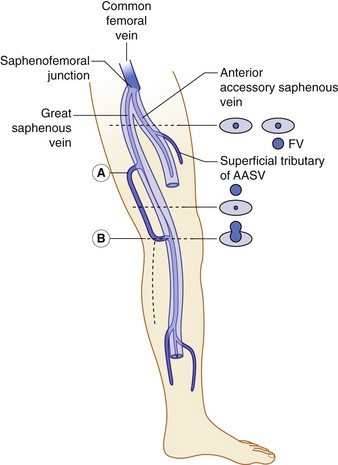
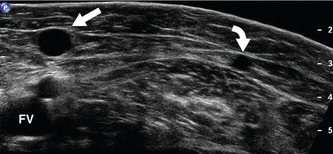
The anatomy of the GSV is shown in Figure 13.3A. The distal GSV is located anterior to the medial malleolus (inner ankle bone), runs up the medial aspect of the calf and thigh, and is joined by a number of superficial tributaries. The GSV drains into the common femoral vein approximately 2.5 cm below the inguinal ligament at the SFJ. It is important to have a detailed understanding of the anatomy in this area, as there are at least six other tributaries draining to the GSV at the level of the SFJ (Fig. 13.5). These tributaries can be the source of primary or recurrent varicose veins. It is not usually possible to identify all of these tributaries by ultrasound. The anterior accessory saphenous vein (AASV), sometimes called the anterolateral thigh vein, drains flow from the anterior and lateral aspect of the knee and lower thigh and runs across the anterior aspect of the thigh into the SFJ (Fig. 13.3). However, it can sometimes join the GSV at a variable level below the junction. The AASV is usually easy to identify with ultrasound and it is contained within its own fascial compartment (Figs 13.6 and 13.7). In the upper thigh, it runs in the same line as the femoral vein: this is referred to as the alignment sign and helps to distinguish the AASV from the GSV that lies medial and posterior to the AASV (Fig. 13.7). The posterior accessory saphenous vein also runs in a facial compartment and drains flow from the posteromedial and posterior regions of the lower thigh and usually joins the main trunk of the GSV in the upper thigh. There are sometimes connections between the thigh extension (TE) of the SSV or Giacomini vein, described later in the chapter.
The SSV, saphenopopliteal junction, and Giacomini vein
The anatomy of the SSV is shown in Figure 13.3B.
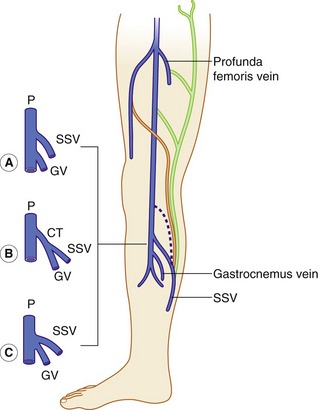
The distal SSV arises posterior to the outer aspect of the ankle (lateral malleolus) and runs up the posterior calf in an interfascial compartment (Fig. 13.4B). In the upper calf the compartment appears as a triangular shape that is defined by the lateral and medial heads of the gastrocnemius muscle and the superficial fascia that streches over the intermuscular groove (Cavezzi et al. 2006). It drains to the popliteal vein via the saphenopopliteal junction located superior to the insertion of the gastrocnemius vein. Typically the saphenopopliteal junction is located 2−5 cm above the popliteal knee crease. However, the anatomy can be extremely variable. The saphenopopliteal junction may be located proximal to the popliteal fossa, draining to the above-knee popliteal vein or distal femoral vein (Fig. 13.8). The SSV can insert directly into the gastrocnemius vein or share a common confluence (Fig. 13.8). In addition, it is common to find a TE of the SSV, described by Giacomini in 1873 running in an intrafascial compartment or groove bounded by the semitendinous muscle, long head of biceps, and superficial fascia (Georgiev et al. 2003).
There are a number of terminations for the TE, as shown in Figure 13.8. The TE is often referred to as the Giacomini vein, although strictly this term should only be applied when the vein connects to the GSV. There is sometimes confusion as to whether a posterior thigh vein is the Giacomini vein or merely a superficial posteromedial tributary of the GSV. Generally, the Giacomini vein should appear to run into an intrafascial compartment in the lower thigh before joining the SSV, whereas superficial tributaries of the GSV tend to lie above the superficial fascia. Finally, there are usually intersaphenous veins in the upper calf, running between the GSV and SSV.
Perforators
There are major perforating veins in the GSV and SSV system and these can be variable in their presence, being more numerous below the knee (Fig. 13.3). Large perforators are easy to identify by ultrasound. It is worth noting that some perforators do not connect directly to the main trunks of the GSV or SSV, but communicate via side branches of the main trunks. There are a number of perforating veins associated with the SSV, especially from medial and lateral gastrocnemius veins and soleal veins.
Other superficial veins
There is typically a lateral venous system in the leg but the veins are often very small and difficult to detect. They may represent an embryonic system and are only relevant if there are isolated varicose veins in this area not associated with the GSV or SSV.
Anatomical variations
There are numerous anatomical variations in the lower-limb venous system, and even experienced sonographers will encounter new variations from time to time. Duplicated, or bifid, vein systems are relatively common and mainly involve the femoral vein and popliteal vein (Fig. 13.9). A potentially confusing anatomical variation occurs in patients who have a large deep femoral vein in the thigh running as a large trunk between the popliteal vein and the common femoral vein. In this situation, the size of the femoral vein in the thigh can be small when compared with the size of the superficial femoral artery, and this appearance may be mistaken for evidence of venous obstruction. However, good flow augmentation, with a calf squeeze, will be demonstrated in the common femoral vein just below the SFJ. Careful inspection by duplex will normally reveal the larger deep femoral vein. A low-frequency 2–4 MHz curved linear array transducer may be necessary to identify this vein. A very rare anomaly can occur toward the level of the SFJ, with the GSV running between the superficial femoral artery and profunda femoris artery to drain into the SFJ.
Venous valves
Veins contain bicuspid, valves to prevent the reflux of blood to the extremities. There is often a characteristic dilation of the vein at the valve site that can sometimes be seen on the ultrasound image (Fig. 13.10). Venous valves are able to withstand high degrees of back pressure, typically in excess of 250–300 mmHg. The number of valves in each venous segment varies among individuals, but there are more valves in the distal veins than in the proximal veins, as they have to withstand higher hydrostatic pressures. The inferior vena cava and common iliac vein have no valves, and the majority of the population have no valves in the external iliac or common femoral vein. There is usually a valve at the proximal end of the femoral vein and an average of three to four valves along the length of the femoral and popliteal vein to the level of the knee, although the number can be inconsistent. In most people there is a valve in the below-knee popliteal vein that is sometimes referred to as the ‘gatekeeper,’ as it prevents venous reflux into the proximal calf. The deep veins in the calf contain numerous valves. The GSV and SSV contain approximately 8–10 valves along their main trunks (Browse et al. 1999).
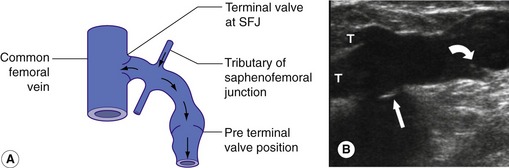
The configuration of the valves at the SFJ is important to consider as there is typically a terminal valve located at the junction and a preterminal valve that is positioned in the GSV, 3–5 cm distal to the terminal valve. Tributaries of the SFJ join between these two points (Fig. 13.11). This explains how it is possible for the junction to be competent but for reflux to occur across the preterminal valve into the proximal GSV due to flow from the tributaries (Caggiati et al. 2004) (Fig. 13.11A). A similar pattern can exist in the SSV. In addition, there are normally valves protecting many perforating veins so that flow is directed from the superficial venous system to the deep system. In very rare cases, patients may have absent venous valves due to congenital valve aplasia (Eifert et al. 2000). This can be the cause of deep venous reflux in the very young patient.
Flow patterns in the venous system
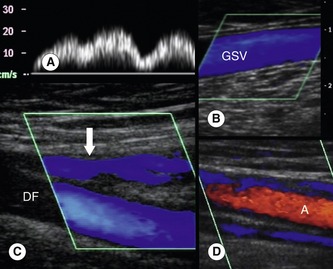
The flow patterns in normal deep veins are described in Chapter 5. The venous flow patterns in the superficial veins can vary, depending on patient position and external factors, such as ambient temperature. Normally there should be no, or very little, spontaneous flow in the GSV and SSV when the patient is standing or sitting. If the ambient temperature in the room is high, vasodilation may result in increased flow in the superficial veins. Evidence of high-volume spontaneous or continuous flow in the superficial veins at rest should be treated with suspicion, as this may indicate obstruction of the deep veins or could be due to infection, such as cellulitis (Fig. 13.12).
VENOUS DISORDERS AND TREATMENT
Deep-vein thrombosis is covered in Chapter 14.
Classification of chronic venous disorders
The CEAP (Clinical−Etiology−Anatomy−Pathophysiology) classification system for venous disorders was developed by an ad hoc committee of the venous forum (Eklöf et al. 2004). It has been developed to facilitate the use of consistent terminology and diagnosis within the clinical and scientific community. The clinical classes are shown in Table 13.1. Therefore, a patient with a painful venous ulcer due to primary venous disease that included great saphenous and popliteal vein reflux, with perforating vein reflux in the calf would have a basic CEAP classification of C6,s,Ep,Aspd,Pr. In advance CEAP, the classification would be C2,6,s,Ep,As,p,d,Pr2,3,18,14.
Table 13.1 CEAP (Clinical–Etiology–Anatomy–Pathophysiology) classification
| CLINICAL CLASSIFICATION | |
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectasia or reticular veins |
| C2 | Varicose veins |
| C3 | Edema |
| C4a | Pigmentation or eczema |
| C4b | Lipodermatosclerosis or atrophie blanche |
| C5 | Healed venous ulcer |
| C6 | Active venous ulcer |
| S | Symptomatic, including ache, pain, tightness, skin irritation, heaviness, and muscle cramps, and other complaints attributable to venous dysfunction |
| A | Asymptomatic |
| ETIOLOGIC CLASSIFICATION | |
| Ec | Congenital |
| Ep | Primary |
| Es | Secondary (postthrombotic) |
| En | No venous cause identified |
| ANATOMIC CLASSIFICATION | |
| As | Superficial veins |
| Ap | Perforator veins |
| Ad | Deep veins |
| An | No venous location identified |
| PATHOPHYSIOLOGIC CLASSIFICATION | |
| Basic CEAP | |
| Pr | Reflux |
| Po | Obstruction |
| Pr,o | Reflux and obstruction |
| Pn | No venous pathophysiology identifiable |
| ADVANCED CEAP: SAME AS BASIC CEAP, WITH THE ADDITION THAT ANY OF 18 NAMED VENOUS SEGMENTS CAN BE USED AS LOCATORS FOR VENOUS PATHOLOGY | |
| Superficial veins | |
| 1 | Telangiectasia or reticular veins |
| 2 | Great saphenous vein above knee |
| 3 | Great saphenous vein below knee |
| 4 | Small saphenous vein |
| 5 | Nonsaphenous veins |
| Deep veins | |
| 6 | Inferior vena cava |
| 7 | Common iliac vein |
| 8 | Internal iliac vein |
| 9 | External iliac vein |
| 10 | Pelvic: gonadal, broad ligament veins, other |
| 11 | Common femoral vein |
| 12 | Deep femoral vein |
| 13 | Femoral vein |
| 14 | Popliteal vein |
| 15 | Crural: anterior tibial, posterior tibial, peroneal veins (all paired) |
| 16 | Muscular: gastrocnemial, soleal veins, other |
| Perforating veins | |
| 17 | Thigh |
| 18 | Calf |
Modified from Eklöf et al. (2004).
Varicose veins
The cause of varicose veins is uncertain, but there is evidence that increased age, female gender, and pregnancy are risk factors (Callam 1994).
It is important to identify the supply to the varicose areas and the level of incompetence in the GSV or SSV. This can be highly variable but frequently involves reflux from the saphenofemoral or saphenopopliteal junctions. In some situations varicose veins may be independent of the GSV or SSV systems, such as the lateral thigh. Much debate has surrounded the development of varicose veins, but it appears that incompetence in the main trunks develops distally, in the lower leg, and progresses in a proximal direction over time. This is contrary to traditional teaching, which proposed that incompetence developed at the SFJ, leading to progressive valve failure in a descending direction. The model of progressively ascending valve failure would also explain why it is possible to observe segmental GSV reflux in the lower thigh but competence of the vein in the upper thigh and at the SFJ (Abu-Own et al. 1994). Some patients with superficial varicose veins may also have coexisting deep venous insufficiency.
Skin changes and venous ulcers
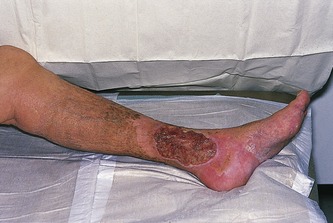
A serious complication of superficial or deep venous insufficiency is the development of chronic venous hypertension in the lower limb, resulting in venous ulceration (Fig. 13.13). Risk factors associated with ulceration include post-thrombotic syndrome, obesity, immobility, and arthritic conditions, which cause reduced movement of the ankle joint, leading to failure of the calf muscle pump. It is important to note that some ulcers that may appear to be venous in origin are caused by other conditions, such as vasculitis, rheumatoid arthritis, or skin disorders. The underlying cause of ulceration is still unclear but is thought to involve changes in the microcirculation of the skin and subcutaneous tissues in response to local venous hypertension. The venous hypertension causes an increase in venular and capillary pressure, leading to local edema and reduced reabsorption of proteins and fluid from the interstitial tissue spaces. This is combined with damage to the capillary walls, which may cause localized tissue hypoxia. Leakage of red blood cells across the damaged capillary wall and into the interstitial tissue spaces produces the brown pigmentation associated with many ulcers. This is due to hemosiderin deposition caused by the breakdown of the red blood cells. Venous ulcers are usually reasonably shallow and vary in size, and in some cases they may be circumferential around the calf. They frequently become infected with different types of bacteria and can be extremely painful.
Historically, it was thought that venous ulceration was primarily due to deep venous insufficiency following valve failure, postthrombotic syndrome, or failure of the calf muscle pump, resulting in deep venous hypertension. However, later studies (Scriven et al. 1997; Magnusson et al. 2001) demonstrated that a significant number of patients with ulceration have superficial reflux alone, with the deep veins being competent. Therefore, ligation of the relevant superficial vein junction, with or without stripping of the superficial vein, results in the healing of the majority of ulcers due to the reduction in venous hypertension. The role of perforator ligation remains controversial, but there is evidence that chronic venous insufficiency is associated with an increase in the number and diameter of medial calf perforators (Stuart et al. 2000). Presurgical marking of incompetent perforators may be performed with the aid of the duplex scanner.
Varicose ulcers caused by superficial incompetence alongside significant deep venous insufficiency are not usually treated by the ligation or stripping of superficial varicose veins, as the underlying deep venous hypertension will not be corrected. Instead, the use of compression bandaging, which reduces edema and venous hypertension, has proved to be an effective method of healing ulcers. Different grades of compression bandaging can be used depending upon the clinical situation (Lambourne et al. 1996). However, an ABPI of ≥0.8 is required for the application of four-layer compression dressings, in order to avoid arterial compromise in the tissues under the bandaging. This can be a serious complication and can lead to limb loss in extreme cases.
Treatment of superficial venous disorders
A range of treatment options is available depending on the severity of the condition (Browse et al. 1999). Conservative treatment is with compression hosiery. Thread veins can be treated by microinjection sclerotherapy, followed by local compression to occlude the vein. They can also be treated by local laser therapy. Larger varicose veins can be treated by a variety of methods, including foam sclerotherapy, open surgery, or endovenous ablation. Some patients undergo combined procedures to achieve optimum results. In the case of open surgery for primary GSV incompetence, the SFJ and tributaries are ligated at the groin, and the main trunk stripped with a vein stripper to knee level or below. Surgery of the SSV normally involves ligation of the saphenopopliteal junction. Some surgeons strip the vein, but others leave it intact to avoid injury to the sural nerve, which is closely associated with the vein. Some surgeons also ligate large perforators, and these can be marked preoperatively with the aid of duplex scanning. Any remaining veins are then removed or avulsed using small microincisions. It is worth observing some varicose vein surgery as it gives a better appreciation of the anatomy seen during duplex examinations.