Blunt Trauma to Duodenum
√ retroperitoneal hematoma
√ stranding of retroperitoneal fatty tissue
√ thickening of duodenal wall
1. Duodenal contusion
√ edema / hematoma of the duodenal wall
√ intramural gas accumulations
√ focal duodenal wall thickening > 4 mm
Rx: conservative
2. Hematoma of the duodenal wall
3. Duodenal perforation and disruption
√ retroperitoneal collection of contrast (infrequent)
√ extraluminal gas
√ lack of continuity of duodenal wall
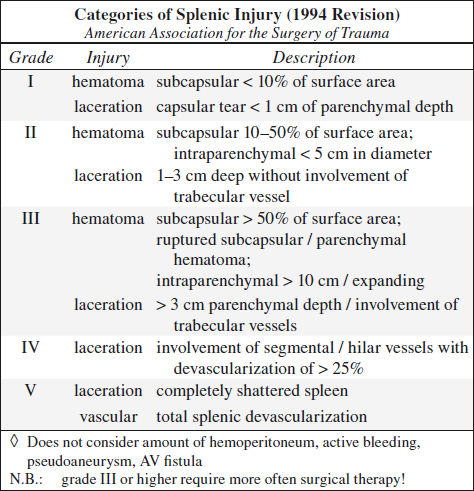
Blunt Trauma to Spleen (40%)
◊ Most frequently injured intraperitoneal organ in blunt abdominal trauma (40% of abdominal organ injuries)
Associated with: other solid visceral / bowel injuries (29%); lower rib fractures in 44%, injury to left kidney in 10%, injury to left diaphragm in 2%
◊ 20% of left rib fractures have splenic injury!
◊ 25% of left renal injury have splenic injury!
Technique: scanning delay of 60–70 sec to avoid heterogeneous splenic enhancement
CECT (95% accurate):
◊ CT not reliable to determine need for surgical intervention!
√ hemoperitoneum ← disruption of splenic capsule
√ “sentinel clot” (= area of > 60 HU adjacent to spleen) as sensitive predictor of injury = perisplenic hematoma
√ active extravasation:
√ high-attenuation blush (80–370 HU)
√ focal high-attenuation area in / emanating from injured splenic parenchyma
√ growing larger with time (delayed phase imaging!)
N.B.: active extravasation of contrast material requires emergent surgery in 83–93%
√ mottled parenchymal enhancement = contusion
√ hypoattenuating line connecting opposing visceral surfaces = linear parenchymal defect = splenic laceration:
√ almost always associated with hemoperitoneum
√ crescentic region of low attenuation along splenic margin flattening / indenting / compressing the normal parenchyma = subcapsular hematoma
√ round hypodense inhomogeneous region ± hyperdense clot = intrasplenic hematoma
√ hypoattenuating hematoma with complete separation of splenic fragments = laceration traversing two capsular surfaces = splenic fracture
√ multiple lacerations = “shattered spleen”
US:
√ hyperechoic intraparenchymal region (= acute hematoma / laceration)
√ anechoic intralesional collection (= brisk hemorrhage)
√ diffusely heterogeneous parenchymal pattern containing hyper- and hypoechoic areas (= extensive splenic injury)
√ loss of normal organ contour ← perisplenic clot
Sequelae:
(1) scar / fibrosis
(2) splenic pseudocyst (20–30 HU)
(3) Vascular injury: pseudoaneurysm, AV fistula
(4) delayed splenic rupture
= hemorrhage > 48 hours after trauma
Prevalence: 0.3–20% of blunt splenic injuries
Time of onset: in 70% within 2 weeks of injury; in 90% within 4 weeks of injury
Prognosis: splenectomy / splenorrhaphy; 80–90% success in nonoperative management
Rx: up to 91% of stable patients can be treated conservatively with observation; transcatheter embolization
◊ Preservation of spleen and its immune function = standard of care
| DDx: | (1) Normal lobulation / splenic cleft (smoothly contoured, medially located) |
(2) Adjacent unopacified jejunum simulating splenic tissue | |
(3) Early differential enhancement of red and white pulp (scan obtained within 20–50 seconds) | |
(4) Perisplenic fluid from ascites / urine / succus / bile / lavage |
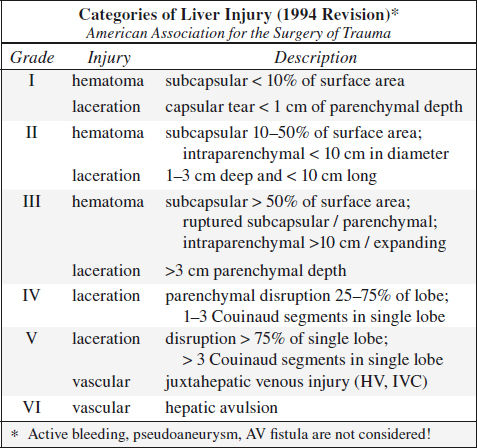
Blunt Trauma to Liver (20%)
Prevalence: 2nd most frequently injured abdominal viscus
Associated with: splenic injury in 45%
• clinical manifestation often delayed by days / weeks
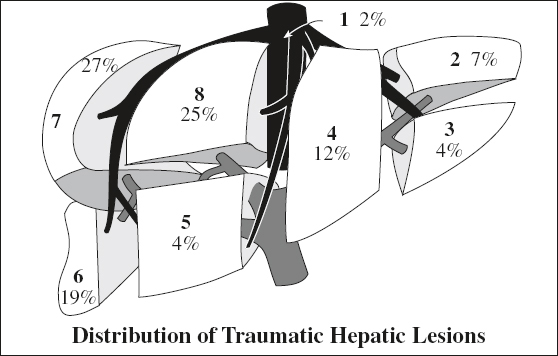
Location: right (posterior segment) > left lobe
Site: perivascular, paralleling right + middle hepatic arteries + posterior branches of right portal vein, avulsion of right hepatic vein from IVC (13%)
◊ Left lobe injuries are more often associated with damage to duodenum, pancreas, transverse colon
CECT:
√ liver laceration = predominantly irregular linear branching / round regions of low attenuation:
(a) superficial ≤ 3 cm deep; (b) > 3 cm in depth
◊ Most frequently identified injury pattern
Associated with:
› retroperitoneal hematoma surrounding IVC ← posterosuperior segment VII laceration of bare area
› hematoma involving adrenal gland
› biloma ← laceration extending into porta hepatis
| DDx: | (1) beam-hardening artifact from adjacent ribs / from air-contrast level in stomach |
(2) Focal fatty infiltration |
√ liver hematoma:
√ hyperattenuating mass in acute phase of 54 (range, 28–82) HU decreasing over time ← clotted blood:
› intraparenchymal hematoma: single / multiple
› subcapsular hematoma: lenticular / elliptical configuration flattening underlying liver margin
Resolution: usually within 6–8 weeks
√ active (potentially life-threatening) liver hemorrhage:
√ focal hyperattenuating area during early phase of 155 (range, 91–274) HU ← extravasated contrast material
Prognosis: 75% become hemodynamically unstable
Rx: angiographic embolization
DDx: focal hyperdense area of 80–350 HU during early phase ← pseudoaneurysm / AV fistula
√ major (life-threatening) hepatic venous injury
= liver laceration or hematoma extending into major hepatic vein / IVC
◊ 3.5 x more frequently associated with arterial bleeding
√ focal / diffuse periportal tracking (in up to 22%)
= areas of low attenuation paralleling portal vein + its branches 2° to
› dissecting hemorrhage / bile
› dilated engorged periportal lymphatics ← ↑ central venous pressure ← vigorous IV fluid administration / tension pneumothorax / pericardial tamponade
√ flat IVC
= AP diameter < ¼ of IVC width not caused by external compression
Cause: hypovolemia, poor fluid resuscitation, shock
√ hypodense wedge extending to liver surface = focal hepatic devascularization
√ hemoperitoneum ← violation of liver capsule with inability of liver veins to contract
√ intrahepatic / subcapsular gas, usually ← necrosis
US:
√ localized area of increased intraparenchymal echogenicity (= acute hematoma / laceration)
√ widespread heterogeneous liver echogenicity + absence of normal vascular pattern (= global parenchymal injury)
Cx: in 5–23%
(1)delayed hemorrhage(2–6%)
(2)abscess (0.6–4%) ← superinfection of hematoma / biloma / devascularized hepatic parenchyma
(3)pseudoaneurysm (1%) → hemobilia, melena, hematemesis (decompression into biliary system)
(4)bile peritonitis
(5)biliary fistula: external (to skin / hollow viscus), internal (to intestine / bronchus), biliovascular (to hepatic artery, portal / hepatic vein)
Rx: conservative treatment in up to 80% in adults + 97% in children; transcatheter embolization
Prognosis: healing in 1–6–15 months; 4–12% mortality
Blunt Trauma to Gallbladder (2%)
Associated with: injury to liver (91%), duodenum (54%), spleen (54%)
√ pericholecystic fluid (extraperitoneal location of GB)
√ free intraperitoneal fluid
CECT:
√ blurred contour of GB
√ focal thickening / discontinuity of GB wall
√ intraluminal enhancing mucosal flap
√ blood within GB lumen = attenuation > 50 HU
√ mass effect on adjacent duodenum
√ collapsed GB ← GB rupture
√ focal periportal tracking ← GB rupture
US:
√ focal hypoechoic thickening
√ echogenic mass within GB lumen
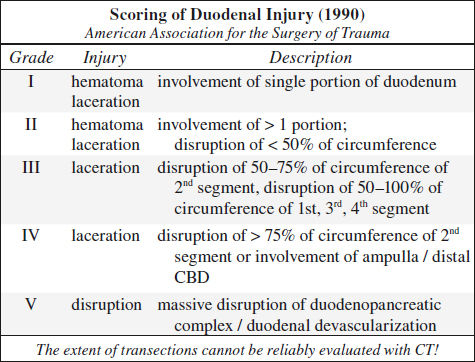
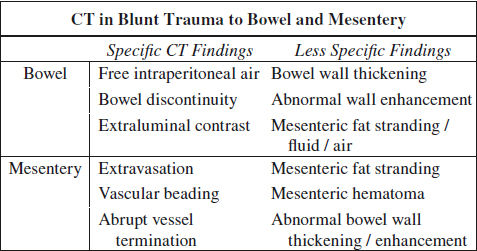
Blunt Trauma to GI Tract (5%)
◊ 3rd most common type of injury from blunt trauma to abdominal organs
Cause in children: MVA (lap belts), bicycle handle bar, child abuse
May be associated with: Chance fracture; traumatic hernia (disruption of the rectus abdominis m.)
Mechanism:
(1) crush / compression injury: direct force; near spine
(2) burst injury: sudden increase in intraluminal pressure
(3) shear injury: rapid deceleration at points of transition between mobile and fixed bowel portions
Location: jejunum distal to ligament of Treitz > duodenum > ascending colon at ileocecal valve > descending colon > distal ileum near ileocecal valve
• Classic triad (in only 33%):
• abdominal pain + tenderness (100% sensitive)
• abdominal rigidity; absent / decreased bowel sounds
• ↑ temperature + heart rate; ↓ urine output over 24 hours
• lap belt ecchymosis (not highly correlated)
• Lavage: 90% sensitive for hemoperitoneum → compromises interpretation of CT exam
N.B.: clinical signs + symptoms may be delayed for 24 hours (increasing mortality to 65%)
US:
√ nonspecific free intraabdominal fluid (86% sensitive, 98% specific)
NECT:
√ abdominal wall injury: SQ fat stranding (“seat belt” sign) ← hematoma ← tear
√ extraintestinal free air (30–60% sensitive, 95% specific):
√ intraperitoneal air: small gas bubbles anteriorly near liver / trapped within leaves of mesentery (with small bowel perforation) / porta hepatis
√ retroperitoneal air (with disruption of duodenum / rectum / colon)
DDx of free air:
◊ Most bowel perforations have no free gas due to:
(a) spontaneous seal of perforation
(b) developing ileus → no passage of gas
(c) rapid reabsorption of small gas collections
√ intramural air
√ hypodense free fluid (90–100% sensitive, 15–25% specific), particularly in interloop location ← perforation
DDx: parenchymal organ injury / osseous injury / large vessel injury / bladder perforation
√ “sentinel clot” sign adjacent to bowel
CECT (84–94% sensitive, 84–99% accurate):
@ bowel injury (CT 94% sensitive, 88% accurate)
Location: small bowel (proximal jejunum, distal ileum) > colon > stomach
√ extravasation of oral contrast material (8–15% sensitive, 100% specific), densest near perforation
DDx: hyperattenuating blood, extravasating vascular contrast material, leak of contrast material ← ruptured urinary tract
√ focal discontinuity of bowel wall = direct evidence (5–10% sensitive, 100% specific)
√ focal bowel wall thickening > 3 mm (= intramural hematoma [55–75% sensitive, 90% specific] / vascular compromise and inflammation ← spilling of bowel contents):
√ ± intestinal obstruction / ileus
DDx: lack of bowel distension
√ abnormal bowel wall enhancement (10–15% sensitive, 90% specific):
√ hyperdense contrast enhancement of injured bowel wall ← delayed venous transit time (20%)
√ lack of bowel wall enhancement (13%) ← bowel infarct, highly SPECIFIC
√ duodenal submucosal / subserosal hematoma → gastric outlet obstruction
@ mesenteric injury (CT 96% sensitive, 96% accurate)
√ mesenteric contrast extravasation (17%)
√ mesenteric vascular beading (39%) = change in caliber
√ abrupt termination of mesenteric artery / vein (35%)
√ mesenteric infiltration (70–77% sensitive, 40–90% specific) = haziness + fat stranding = streaky hyperattenuating infiltration / fluid at mesenteric root ← hemorrhage + inflammatory response
DDx: retractile mesenteritis
√ mesenteric rent → internal hernia
√ mesenteric hematoma (39%)
√ mesenteric pseudoaneurysm
Cx: peritonitis, sepsis, hemorrhage
Prognosis: delay in diagnosis by 8–12 hours increases morbidity + mortality from peritonitis + sepsis
Rx: surgery based on clinical assessment alone has a 40% negative laparotomy rate
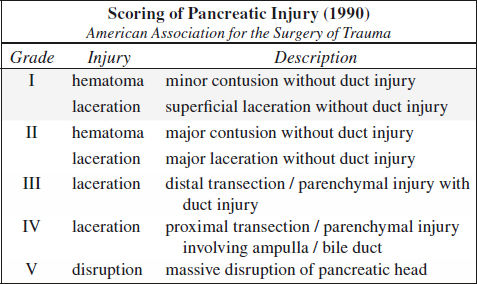
Blunt Trauma to Pancreas (3%)
Mechanism: compression against vertebral column with shear across pancreatic neck
Frequency: < 10% of childhood trauma
Cause: motor vehicle accident (→ compression by seat belt /steering wheel), fall onto handle bars of a bicycle, child abuse
Associated with: injury to liver (47%, typically left lobe), spleen (28%), stomach (42%), duodenum (19%, typically in younger patients), major vessel (41%), kidney (23%)
Classification:
I minor contusion / hematoma, capsule + major duct intact
II parenchymal injury without major duct injury
III major ductal injury
IV severe crush injury
• epigastric/ diffuse abdominal pain, vomiting
• leukocytosis, ↑ serum amylase activity
Location: pancreatic body (65%) > tail / head
CT (70–95% sensitive):
√ within first 12 hours normal CT findings in 20–40% → repeat CT at 24–48 hours
√ posttraumatic pancreatitis:
√ edema / fluid in peripancreatic fat
√ focal / diffuse pancreatic enlargement
√ irregularity of pancreatic contour
√ focal area of low-attenuation / enlargement
(1) contusion = diffuse / localized hypoattenuating area within normally enhancing parenchyma
(2) laceration (actual site of laceration difficult to visualize)
N.B.: pancreatic laceration of > 50% of pancreatic diameter suggests ductal injury. Evaluation of the integrity of the main pancreatic duct remains the critical role of MRCP!
(3) transection (fracture) = hypoattenuating linear findings with separated parenchyma
√ pancreatic hematoma = mixed / slightly hyperattenuating lesion within margins / in contact with parenchyma
√ fluid / blood accumulation:
(a) alongside superior mesenteric artery
(b) in transverse mesocolon / lesser sac
(c) fluid between pancreas and splenic vein (in up to 90%)
√ thickening of anterior pararenal fascia
Morbidity: 11–62%
Mortality: 5%
Rx: I + II conservative management
III + IV need for surgery within 24 hours
endoscopic stent placement for duct injury
Cx: pancreatic fistula (23%), posttraumatic pancreatitis (10%), pseudocyst (5%), pseudoaneurysm, pneumonia, abscess (increases mortality to 20%)
BOERHAAVE SYNDROME
[Herman Boerhaave (1668–1738), professor of clinical medicine, botany and chemistry at the University of Leiden, Netherlands]
= spontaneous emetogenic injury resulting in rupture of esophagus → extrusion of gastric content into mediastinum / pleural space
Frequency: 1÷6,000 persons
Age: middle-aged men (in 50% with history of alcoholism / heavy drinking)
Cause: violent retching ← food bolus impaction
Pathophysiology:
incomplete cricopharyngeal relaxation during sudden increase in intraabdominal pressure while vomiting → abrupt increase in intraluminal esophageal pressure (barotrauma) in the presence of a moderate to large amount of gastric contents → complete transmural disruption of esophageal wall
• Mackler triad:
• episode of severe retching + forceful vomiting
• sudden excruciating chest pain (substernal, left chest, neck, pleuritic, epigastric); subcutaneous emphysema
• odynophagia, tachypnea, cyanosis, fever, shock
• NO hematemesis (blood escapes outside esophageal lumen)
√ rent of 2–5 cm in length
Location: 3–6 cm above diaphragm (= 2–3 cm above GE junction), predominantly in left posterolateral wall
CXR:
√ mediastinal widening
√ air-fluid level within mediastinum
√ extravasation of contrast into mediastinum / pleura
√ pneumomediastinum (single most important plain-film finding), pneumopericardium, subcutaneous air:
√ “V” sign of Naclerio = localized mediastinal emphysema with air between lower thoracic aorta + diaphragm
[Emil A. Naclerio (1915–1985), thoracic surgeon at Harlem and Columbus Hospitals in New York City]
√ pleural effusion on left >> right side / hydropneumothorax
√ subcutaneous emphysema
√ patchy pulmonary infiltrate
Esophagography(modality of choice):
Technique: initially hydrosoluble contrast medium (in 10% falsely negative) followed by barium if negative
√ submucosal collection
√ extravasation of contrast material
√ esophagopleural fistula (most commonly on left)
The use of hydrosoluble contrast material is preferred over barium in suspected esophageal rupture → risk for mediastinitis as a result of irritation caused by barium.
CT:
√ esophageal wall thickening
√ supradiaphragmatic periesophageal air collection
√ mediastinal fluid collection + pneumomediastinum
BRUNNER GLAND HAMARTOMA
[Johann Conrad Brunner (1653–1727), Swiss professor of anatomy and physiology at the University of Heidelberg, Germany]
Terminology:
Brunner gland hamartoma ≤ 5 mm in diameter
Brunner gland hyperplasia > 5 mm in diameter
Prevalence: 1.2% of all gastric polyps; 5% of all duodenal masses
Etiology: response to gastric acid in duodenum → glands protect duodenal epithelium + optimize pH for pancreatic enzyme activity
Histo: diffusely enlarged hyperplastic glands of Swiss cheese appearance and variable amounts of adipose + smooth muscle + lymphoid tissue + sclerosis
Physiology: mucosal + submucosal Brunner glands contain mucous + serous cells → secrete a clear viscous alkaline mucus into crypts of Lieberkühn
Age: manifest in middle age; M÷F = 1÷1
• incidental / symptomatic (abdominal pain)
MORPHOLOGIC TYPES:
1. Diffuse nodular hyperplasia: throughout duodenum
2. Circumscribed nodular hyperplasia: in suprapapillary portion
3. Single glandular adenoma with polypoid tumorlike dimensions
Location: duodenum (70% bulb, 26% 2nd portion, 4% 3rd portion); prepyloric region (distribution of duodenal glands from vicinity of pylorus to proximal ²/³ of duodenum)
Mean size: 2.0 (range, 0.5–6.0) cm; rarely up to 11 cm
UGI:
√ multiple nodular filling defects (usually limited to 1st portion of duodenum) with “cobblestone appearance” (most common finding)
DDx: polyposis syndromes, lymphoid hyperplasia, heterotopic gastric mucosa, nodular duodenitis
√ smooth single mass ± central ulceration
DDx: adenomatous polyp, lipoma, leiomyoma, leiomyosarcoma, lymphoma, ectopic pancreatic tissue, GIST, carcinoid tumor, adenocarcinoma, pancreatic neoplasm, ampullary neoplasm
Endoscopic US:
◊ Guides appropriate depth of endoscopic biopsy!
√ heterogeneous echogenicity with various amounts of solid + cystic components
NECT:
√ isoattenuating relative to pancreas
CECT:
√ hypoattenuating relative to pancreas (portal venous phase)
√ peripheral rim enhancement (of duodenal mucosa)
Cx: GI bleeding (chronic melena, hematemesis), intestinal obstruction, intussusception
BURKITT LYMPHOMA
= highly aggressive B-cell lymphoma usually found in children or immunocompromised adults
[initially described in a 7-year old Ugandan child in 1958 by Denis Parsons Burkitt (1911–1993), Irish surgeon on Medical Research Council in London]
Prevalence: 1–2% of all NHLs; 1–5% of primary gastrointestinal NHLs in adults
◊ Most common (30–50%) type of pediatric NHL in children < 15 years in USA and western Europe.
Origin: undifferentiated small noncleaved B-cell–derived lymphocyte
Histo: uniform deeply basophilic medium-sized cells containing round nuclei with distinct chromatin and multiple nucleoli; characteristic “starry sky” pattern (= scattered macrophages containing apoptotic cellular debris on a basophilic background) at light microscopy; 99% proliferation index
Genetics: translocation of c-Myc oncogene with one of immuno- globulin genes, most frequently t(8;14)(q24;q32)
Growth rate: fastest growing of all human tumors with a doubling time of about 24 hours
Rx: dramatic response to chemotherapy
Prognosis: 90–98% 5-year survival rate in children with localized disease: 75–89% 2-year disease-free survival rate in children with advanced disease; 50–70% survival in adults
Endemic / African Form of Burkitt Lymphoma
Endemic in areas with malaria:
sub-Saharan Africa, New Guinea (exposure to Plasmodium falciparum has a synergistic effect causing a marked decrease in T-cell surveillance)
Incidence in central Africa:
50–80% of all childhood neoplasms
Associated with: Epstein-Barr virus infection in 95% (implicated as B-cell mitogen in oncogenesis); malaria
Age: 3–10 years
@ Mandible > maxilla / facial bones
• jaw mass; exophthalmos (orbital extension)
√ grossly destructive lesion, spicules of bone growing at right angles
√ large soft-tissue mass
@ Other skeleton (multifocal in 10%)
√ reminiscent of Ewing tumor / reticulum cell sarcoma
√ lamellated periosteal reaction around major long bones
Sporadic / American Form of Burkitt Lymphoma
= NONENDEMIC FORM OF BURKITT LYMPHOMA
= typically manifests with bulky disease because of its rapid doubling time
Incidence in Europe + North America:
35–45% of all pediatric NHL; 3% of all childhood tumors
Median age: 8 (range, 6–15) years; ⅓ between 5 and 9 years; unusual in children < 5 years; most frequently in white boys
• paraplegia; NO peripheral leukemia
• Epstein-Barr virus genome found in only a minority
◊ Widespread extraintestinal disease at presentation (mesenteric ± retroperitoneal lymphadenopathy) in 70%!
@ Gastrointestinal tract (22–69%)
• abdominal mass, intestinal obstruction
• acute abdominal complaints (30–40%)
Location: terminal ileum, ileocecal region (Peyer patches), mesentery >> stomach, colon
√ well-defined sharply marginated homogeneous large abdominal and pelvic masses (31–64%):
√ encasement of bowel and mesenteric vessels
√ invading bowel wall → obstruction
√ central necrosis in large tumor
√ ± enlarged abdominal lymph nodes
√ malignant ascites (25%–63%)
√ peritoneal thickening / nodularity (42%) along liver capsule and peritoneal reflections ← intraperitoneal seeding
√ usually intraabdominal extranodal involvement with sparing of spleen
Barium:
√ displacement of bowel loops by a large mass
√ abnormally separated bowel loops ← extensive bowel wall thickening
√ narrowing of distal ileum
US:
√ large hypoechoic masses ± engulfing of bowel / mesenteric vessels
√ cystic central areas ← necrosis
√ omental caking (unusual)
CT:
√ bowel wall thickening / mural masses
√ “sandwich” sign = enhancing vessels surrounded by mildly enhancing confluent mesenteric mass
MR:
√ isointense to muscle on T1WI + T2WI:
√ homogeneous T1 hypointensity
√ heterogeneous intermediate-to-high SI on T2WI
√ intense homogeneous enhancement
√ bright round-to-ovoid lesions > 10 mm in size with restricted diffusion ← lymph node involvement
PET/CT:
√ highly FDG avid
Cx: intussusception, aneurysmal dilatation, perforation
◊ Most common cause of intussusception in children ≥ 4 years
◊ Intussusception and aneurysmal dilatation of bowel suggest lymphoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree