Fig. 1
Biocontinuum of radiation induced acute, subacute, chronic, and late effects in the CNS (with permission from Rubin and Casarett 1968)
2 Functional Anatomy and Histology
2.1 Anatomy
The nervous system is primarily divided into the central nervous system (CNS), which consists of the brain and spinal cord, and the peripheral nervous system (PNS). Gross examination of the CNS reveals two distinct types of tissue (gray and white matter), which are also easily visible on MRI. Gray matter is made up of neuronal cell bodies and their supporting glial cells and is concentrated in the cerebral cortex, cerebellum, and interior of the spinal cord. Within the white matter, clusters of gray matter form islands such as the basal ganglia, thalamus, cranial nerve nuclei, and multiple other critical structures. White matter consists primarily of axons, with glial cells interspersed among the axonal processes, and gains its white color from the axons’ myelin coating.
The brain is further subdivided into the cerebrum, cerebellum, and brainstem (Fig. 2). The cerebrum controls voluntary movement, sensory processing, speech, memory, and cognition, while the cerebellum is involved in the integration of motor and sensory function as well as balance and motor coordination. The brainstem controls involuntary vital functions such as respiration, plays a role in the regulation of consciousness and the sleep/wake cycle, and contains the nuclei of cranial nerves III–XII. All neural pathways between the brain and spinal cord also pass through the brainstem. The spinal cord is divided craniocaudally into 31 spinal nerves which carry motor and sensory innervation to the entire body, with the exception of the territories in the head and neck supplied by the 12 cranial nerves. The spinal cord is also functionally separated in the axial plane into ascending (sensory) and descending (motor) pathways.
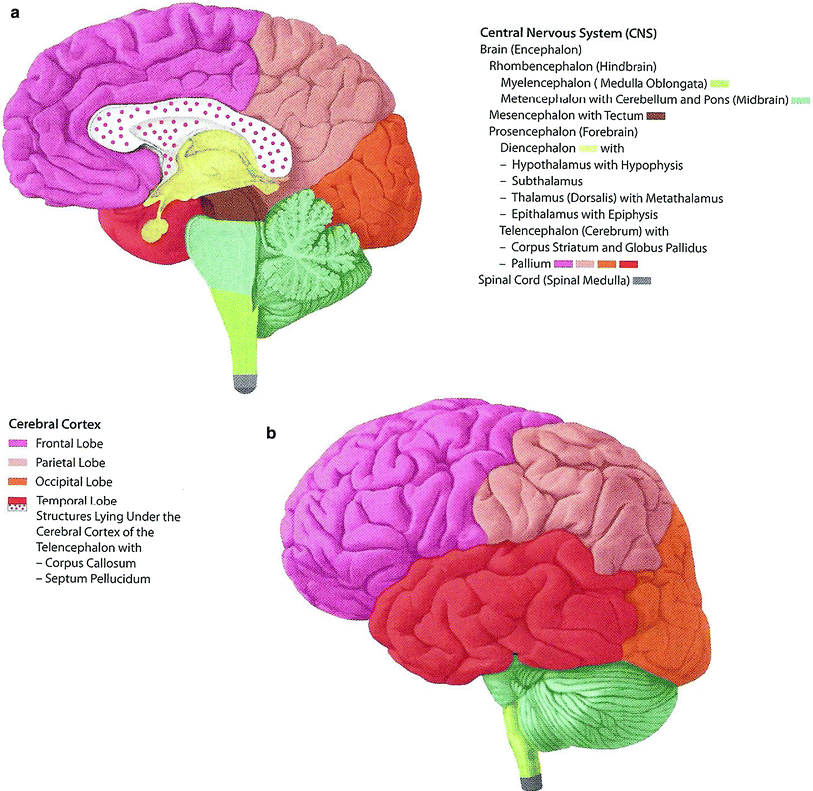

Fig. 2
Different regions of the brain are shown. a Medial view: Median sagittal section; b Left lateral view (with permission from Tillman 2007)
The peripheral nervous system is comprised of all other neural structures outside the spinal cord and includes the somatic nervous system (supplying voluntary motor control to the muscles and returning sensory information to the spinal cord and brain) and the autonomic nervous system (responsible for regulation of involuntary functions such as heart rate, respiration, blood pressure, and digestion).
2.2 Histology
The neuron is well established as the primary structural and functional cell of the nervous system. A variety of glial cells exist as “support cells” in both the central and peripheral nervous system. Oligodendrocytes and Schwann cells are the myelinating cells of the CNS and the PNS, respectively. Our understanding of the role of astrocytes, the most numerous type of glial cells, continues to evolve, but they have an established function as part of the blood–brain barrier (BBB), as mechanical support cells within the CNS, in the metabolism of neurotransmitters and ions such as calcium and potassium, and as the main mediators of gliosis, the response to injury in the brain parenchyma. Ependymal cells line the ventricles of the brain as well as the central canal of the spinal cord and produce cerebrospinal fluid (CSF). Microglia, derived from a monocytic (hematopoietic) cell lineage, are the primary phagocytes of the CNS and play a role in acute brain injury (Fig. 3a, b).
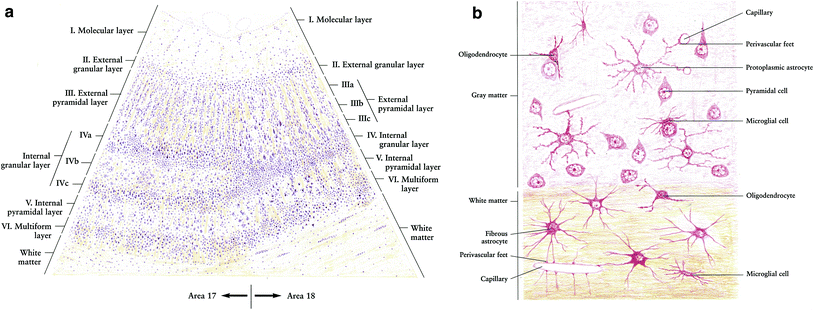

Fig. 3
Histology. a Low power magnification. b High power magnification of the human cerebrum with different cell types marked (with permission from Zhang 1999)
Although neurons themselves are likely incapable of mitotic division, the adult human brain has recently been established to have a neuron-generating stem cell compartment, contradicting previously held dogma. Neurogenesis has been constitutively demonstrated within the dentate gyrus of the hippocampus as well as the subventricular zone and olfactory bulb, and a population of pluripotent neural stem cells (capable at least in vitro of dividing into neurons, astrocytes, and oligodendroglia) exists throughout the brain and spinal cord. Regenerative neurogenesis in response to injury has been demonstrated in the corticospinal tract, neocortex, and striatum. These cell populations are the subject of intense research (Emsley et al. 2005).
3 Biology, Physiology, and Pathophysiology
3.1 Radiobiology of the Central Nervous System
The pyramidal cells are the major neurons of the brain. They are considered post-mitotic cells and as such are unable to be replaced. However, there is evidence of a stem cell region in the brain, located in the hippocampus, which may be capable of regenerating neurons (as illustrated in Fig. 4).
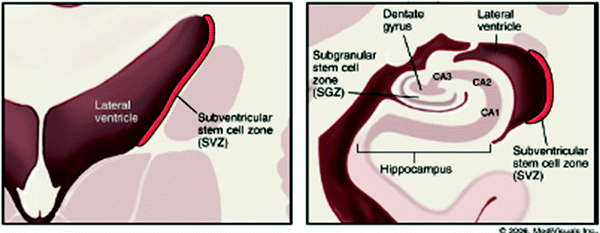

Fig. 4
Distribution of stem cells in the adult human brain (adapted with permissions from Barani et al. 2007)
Classical radiobiology teaches that CNS tissue is a “late-responding” tissue, characterized by a low α/β ratio, delayed manifestation of radiation injury, and extreme variability in response as the fraction size is altered (Hall 2006a). Recovery of neural tissue from radiation injury was predicted by this model to be extremely limited. This has been contradicted by a landmark study in rhesus monkeys published by Ang et al. (2001). Rhesus monkeys were reirradiated at varying doses (44 Gy followed by either 57.2 or 66 Gy) at varying time intervals (1 or 2 years). The animals were then observed for up to 66 months. Histologic studies of the monkey spinal cords suggested significant repair at one year, with recovery continuing to increase through the third year following reirradiation. A dose-response relationship predictive for myelopathy was also observed. From these data, the authors extrapolated conservative estimates of human spinal cord recovery from myelopathy, following an initial dose of 45 Gy, as 50, 60, and 65–70 % at 1, 2, and 3 years, respectively.
Structural differences between the brain and spinal cord result in a major difference in radiation tolerance between the two tissues. Within the brain, islands of eloquent tissue are intermingled with large areas of brain parenchyma which can be significantly damaged or even removed without compromising essential functions. Consequently, many areas of the brain can tolerate high doses of focal radiation without the development of catastrophic damage. In contrast, the spinal cord is a tightly compacted cable of tissue, nearly all of which is functional. Transection or damage to one segment of the spinal cord results in loss of all downstream function of the cord, which has important implications for radiation dose prescription and treatment planning (Fig. 5).
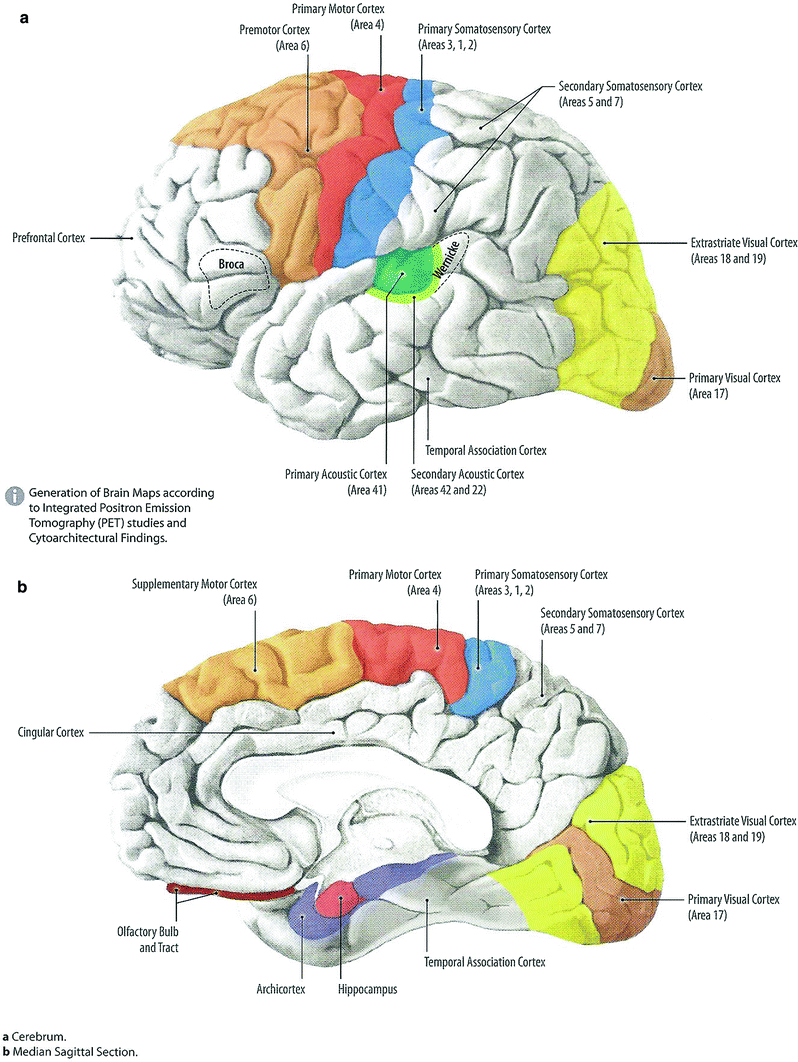

Fig. 5
Physiology: coarse distribution of functions within the brain, a Left lateral view; b Medial view of the right section (with permission from Tillman 2007)
3.2 Pathology and Pathophysiology of Radiation Damage Within the Nervous System
No discrete lesion is pathognomonic for radiation injury to the CNS, which is classically associated with pathologic changes common to most other mechanisms of CNS injury, including demyelination, malacia (decrease in white and gray matter volume), gliosis (scarring), and vascular damage. Foci of liquefactive necrosis associated with significant edema and gliosis may develop in areas receiving high doses of radiation. Figure 6a, b illustrates key features of radiation necrosis. Glial and endothelial cells appear to be the key target cells for radiation damage in the CNS. Because adult neurons are not actively dividing cells, radiation damage to neurons at typical therapeutic doses is therefore unlikely to contribute significantly to CNS toxicity. However, increasing evidence suggests that neural stem cells, an actively dividing cellular compartment, may be subject to radiation damage and play a significant role in late radiation toxicity, particularly with respect to neurocognitive effects.
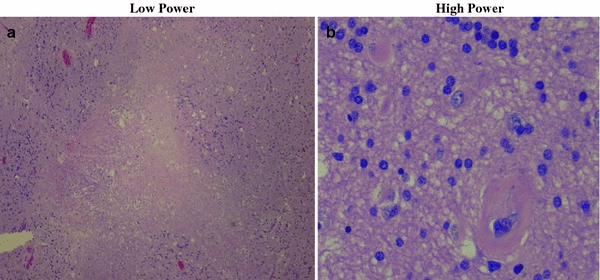

Fig. 6
Radiation Necrosis—Histology. Low power image on the left demonstrates central necrosis with surrounding hypercellular gliosis. High power magnification on the right demonstrates hypercellularity, arteriosclerosis (5 o’clock), and scattered reactive astrocytes, a Low power; b High power
Clinically, radiation toxicity in the CNS is divided into three phases (acute, early delayed, and late), which correlate with different pathophysiologic mechanisms (Kim et al. 2008). In the acute phase, acute inflammation related to cytokine activity and disruption of the BBB dominates. Acute radiation toxicity within the CNS is characterized by headache, fatigue, and, in severe cases, signs of increased intracranial pressure. Transient demyelination is thought to be responsible for early delayed reactions in the CNS. The primary manifestation of early delayed CNS reaction is the somnolence syndrome, which is seen most frequently in children who receive whole brain radiation and intrathecal methotrexate; it typically occurs approximately 6–12 weeks following the completion of whole brain radiation therapy. The hypersomnolence is typically self-limited and its correlation with the development of late neurotoxicity is controversial (Ch’ien et al. 1980; Berg 1983). The manifestations of late neurotoxicity (developing at least 3 months after radiation exposure) are highly variable, ranging from subtle cognitive deficits to severe encephalopathy associated with diffuse white matter damage. Radiation necrosis, variably associated with cerebral edema and focal neurologic deficits, may develop in areas of the brain receiving high (>60 Gy) radiation doses. Late neurotoxicity is mediated by a combination of vascular lesions, cytokine-induced tissue damage, impaired neurogenesis, and reactive oxygen species.
One of the most consistent features of late radiation damage in the CNS is white matter damage (necrosis and demyelination). Oligodendrocytes are responsible for myelinating neurons and appear to be the most radiosensitive glial cells (Barbarese and Barry 1989; Vrdoljak and Bill 1992). Damage to oligodendroglia was thus hypothesized to be the primary mechanism of radiation-induced demyelination. However, damage to endothelial tissue within the CNS appears to play a key role in post-radiation demyelination. This was elegantly demonstrated by a boron-neutron-capture experiment in which the borated compound (BSH) was unable to cross the BBB. Due to the extremely short range of the alpha particles generated by boron neutron capture therapy, the endothelium was selectively irradiated while brain parenchyma was spared. White matter necrosis and demyelination were nonetheless observed, suggesting that glial cells are not the primary target cells in the development of these lesions (Coderre et al. 2006).
An increase in vascular endothelial growth factor (VEGF) production, triggered by BBB disruption, appears to play a key role in the pathogenesis of white matter lesions. In rodent models, radiation damage to the BBB occurs in two phases. Acute apoptosis of endothelial cells is observed within 24 h of radiation, with regeneration of the endothelium complete approximately 14 days after a single fraction of radiation is administered (Li et al. 2006). The late phase of BBB disruption is associated with increasing vascular permeability beginning approximately 3 months after radiation (in the mouse model) (Yuan et al. 2006). Because VEGF itself causes increased vascular permeability, a positive feedback loop is created which ultimately results in significant local edema, inflammation, and hypoxia. Although VEGF levels eventually rise to a level sufficient to trigger angiogenesis, the structure of the BBB in irradiated areas does not return to normal. The loss of normal endothelium is thought to contribute significantly to the development of late white matter necrosis. Anti-VEGF therapies such as bevacizumab are the subject of active investigation as possible modulators of this late response (Gonzalez et al. 2007).
Reactive oxygen species are responsible for approximately 2/3 of X-ray induced DNA damage. Although radiation-generated ROS are themselves short-lived, radiation damage is associated with a prolonged ROS cascade in the damaged normal tissue and chronic oxidative stress. A variety of mechanisms contribute to chronic oxidative stress in irradiated areas. In areas of the CNS which have been damaged by radiation, the BBB is disrupted and the production of pro-inflammatory cytokines (e.g., TNF-α, INF-γ, ICAM-1) is upregulated (Belka et al. 2001). Activated leukocytes as well as CNS microglia are recruited to the area and release large quantities of ROS as they participate in the local inflammatory reaction. Neuronal excitotoxicity is also associated with the release of ROS, as is chronic hypoxia resulting from damage to small blood vessels.
As noted above, certain areas of the brain (primarily the hippocampal dentate gyrus and the subventricular zone) retain constitutive neurogenic stem cell activity throughout life. Memory and learning abilities appear to be correlated with stem cell activity in these regions, at least in available rodent models, and damage to NSC’s in irradiated adults is likely partly responsible for post-radiation neurocognitive deficits (Barani et al. 2007). Neurogenic stem cells appear to be significantly radiosensitive, (Peissner et al. 1999) with rapid and prolonged loss of cell population in the stem cell compartment following radiation (Tada et al. 2000). Juvenile rats have a higher density of active NSC’s and thus appear to be at higher risk for neurocognitive sequelae of brain radiation (Fukuda et al. 2005). This correlates well with the inverse relationship between age at irradiation and the severity of cognitive deficits observed clinically in humans.
4 Clinical Syndromes
4.1 Clinical Syndromes
Exposure of the CNS to radiation results in a variety of clinical manifestations. In an attempt to standardize the evaluation and reporting of neurotoxicity, formal scoring tables for the evaluation and description of acute and late neurotoxicity have been developed. Table 1 summarizes the Late Effects Normal Tissue Task Force–Subjective, Objective, Management, and Analytic (LENT–SOMA) and the National Cancer Institute CTC (V.4) Common Terminology Criteria for Adverse Events (CTCAE) grading systems for neurotoxicity. The clinical expression depends on a host of factors, including total dose, fraction size, treated volume, treatment time, and age of the patient. Other factors contribute to CNS toxicity in many patients undergoing radiotherapy to the brain and spinal cord. These include surgery, medications (e.g., steroids, opioids, benzodiazepines, anticonvulsants), chemotherapy, and pre-existing medical comorbidity). The importance of recurrent or persistent malignancy as a contributor to neurological and neurocognitive sequelae in patients undergoing radiation therapy for brain tumors also should not be underestimated.
Table 1
LENT–SOMA grading criteria for CNS toxicity (Emsley et al. 2005)
|
Brain
|
Grade 1
|
Grade 2
|
Grade 3
|
Grade 4
|
|---|---|---|---|---|
|
Subjective signs
|
||||
|
Headache
|
Occasional, minimal
|
Intermittent, tolerable
|
Persistent, intense
|
Refractory and excruciating
|
|
Somnolence
|
Occasional, able to work or perform normal activity
|
Intermittent, interferes with work or normal activity
|
Persistent, needs some assistance for self care
|
Refractory, prevents daily activity, coma
|
|
Intellectual deficit
|
Minor loss of ability to reason and judge
|
Moderate loss of ability to reason and judge
|
Major loss of ability to reason and judge
|
Complete loss of reasoning and judgement
|
|
Functional competence
|
Perform complex tasks with minor inconvenience
|
Cannot perform complex tasks
|
Cannot perform complex tasks
|
Incapable of selfcare/supervision, coma
|
|
Memory
|
Decreased short term, difficulty with learning
|
Decreased short term, loss of short term
|
Loss of short and long term
|
Complete disorientation
|
|
Objective signs
|
||||
|
Neurologic deficit
|
Barely detectable neurologic signs, able to perform normal activities
|
Easily detectable neurologic abnormalities, interferes with normal activities
|
Focal motor signs, disturbances in speech, vision, etc.interfering with daily activities
|
Hemiplegia, hemisensory deficit, aphasia, blindness, etc. requires continuos care, coma
|
|
Cognitive functions
|
Minor loss of memory,reason and/or judgement
|
Moderate loss of memory,reason and/or judgement
|
Major intellectual impairment
|
Complete memory loss and/or incapable of rational thought
|
|
Mood and personality changes
|
Occasional and minor
|
Intermittent and minor
|
Persistent and minor
|
Total disintegration
|
|
Seizures
|
Focal, without impairment of consciousness
|
Focal with impairment of consciousness
|
Generalised, tonioclinic or absence attack
|
Uncontrolled with loss of consciousness >10 mn
|
|
Management
|
||||
|
Headache, somnolence
|
Occasional nonnarcotic medication
|
Persistent nonnarcotic medication intermittent low dose steroids
|
Intermittent high dose steroids
|
Parental high dose steroids, mannitol and/or surgery
|
|
Seizures
|
Behavioural modification
|
Behavioural modification and occasional oral medication
|
Permanent oral medication
|
Intravenous anticonvulsive medication
|
|
Cognition, Memory
|
Minor adaptation
|
Psychosocial + educational intervention
|
Occupational and physiotherapy
|
Custodial care
|
|
Analytic
|
||||
|
MRI
|
Focal white matter changes; dystrophic cerebral calcification
|
White matter changes affecting <1 cerebral lobe; limited perilesional necrosis
|
Focal necrosis with mass effect
|
Pronounced white matter changes; mass effect requiring surgical intervention
|
|
CT
|
Assessment of swelling, oedema, atrophy
|
The clinical endpoints are summarized in Table 2 as Focal and Global events. Late CNS toxicity may be broadly, and somewhat arbitrarily, segregated into categories as shown.
Table 2
Representative clinical endpoints: arbitrarily segregated into subgroups as shown
|
Focal
|
Global
|
|
|
Subclinical
|
1. Radiologic abnormality
|
1. Modest declines in IQ
2. Diffuse EEG findings
|
|
Clinical
|
1. Dysmobility of a limb
2. Focal loss of sensation
3. Aphasia
4. Visual field cut
5. Inability to form new memories
|
1. Clinically evident neurocognitive problems
2. Mental retardation
|
4.1.1 Cerebrovascular Syndrome
Acute exposure to high total body doses (≥20–30 Gy) causes the cerebrovascular syndrome. Fatal within 24–48 h, this syndrome is associated with systemic loss of vascular permeability and the rapid onset of cerebral edema and multiorgan failure. The few reported human cases have been associated with prodromal symptoms including fever, confusion, and weakness. These are followed by a brief (5–6 h) latent period where recovery of mental status and blood pressure may occur. This latent phase rapidly progresses to the final stage of the cerebrovascular syndrome, associated with fever, diarrhea, refractory hypotension, and progressive cerebral edema causing worsening mental status and death (state when death usually occurs) (Hall 2006b; Waselenko et al. 2004).
4.1.2 RT-Induced Neurocognitive Deficits in the Adult Population
Brain radiation induces late cognitive changes in the adult brain as well. The precise evaluation of these changes is complicated by a number of factors. Many patients with disorders (brain metastases, malignant glioma) requiring brain RT have a limited lifespan, and do not survive long enough to develop late neurocognitive changes, which can develop years after cranial RT. Surgery, chemotherapy, medications, and disease recurrence also cause neurotoxicity, further complicating the precise evaluation of radiation’s contribution to cognitive deficits (see above). Finally, accurate assessment of neurocognitive deficits requires serial neurocognitive testing for years following radiation, which frequently is not feasible (Crossen 1994). The RTOG has investigated a battery of previously validated neurocognitive tests which can be administered in 45 min, shown in Table 3 (Regine et al. 2004).
Table 3
Battery of neurocognitive tests assessed in RTOG BR-0018
|
Test name
|
Functions assessed
|
|---|---|
|
Mini-mental status exam (MMSE)
|
Memory, attention, cognition
|
|
Hopkins verbal learning test (HVLT)
|
Memory
|
|
Verbal fluency/Controlled word association test (COWAT)
|
Executive functioning, verbal learning, working memory, and vocabulary
|
|
Ruff 2 and 7
|
Selective attention
|
|
Trail making test A and B (TMT-A, TMT-B)
|
Focused attention and speed performance
|
|
Profile of mood states short form (POMS)
|
Tension, depression, anger, vigor, fatigue, confusion
|
Radiation-induced dementia associated with diffuse leukoencephalopathy is characterized by depression, emotional liability, and deficits in memory and attention which progress to gait disturbance and incontinence in approximately 80% of patients, as shown in Fig. 7. An important differential diagnosis is normal-pressure hydrocephalus. Spontaneous improvement is rare. The only available therapy is supportive care, and the time to death after developing symptoms of radiation-induced dementia ranges from 1 month to 2 years (Keime-Guibert et al. 1998). The use of concurrent chemotherapy increases the incidence of radiation-induced dementia (Frytak et al. 1989).
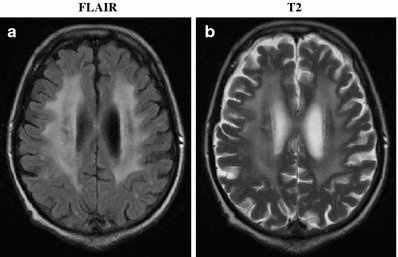

Fig. 7
Radiation-Induced Diffuse White Matter Abnormality: a 73 year-old man presented with a single brain metastasis from colon cancer. He received 37.5 Gy in 2.5 Gy fractions of whole brain radiation followed by radiosurgery boost. He recurred with multiple new enhancing lesions (all subcentimeter) suspicious for metastasis. He received 21.6 Gy in 1.8 Gy fractions of repeat whole brain radiation. Six months later, his MRI revealed diffuse white matter changes on both FLAIR (a) and T2 (b) sequences
Typically, however, neurocognitive deficits in adults are subtle, and outcomes are generally favorable with modern fractionation schemes (Brown et al. 2003). Armstrong et al. reported on a series of young patients with supratentorial, favorable-histology brain tumors who received partial brain RT with doses ranging from 46 to 63 Gy (Armstrong et al. 1995). Serial neurocognitive testing (at baseline and at regular intervals up to 3 years after completing RT) was performed; the RT patients were also compared with a group of age-matched controls. Patients experienced “subtle early-delayed memory changes [that were] followed by a rebound of ability” by 1 year after completing RT. Disease control (Regine et al. 2001) and pre-treatment cognitive function (Brown et al. 2001) also appear to be important predictors of post-RT cognitive status. Temporal lobe radionecrosis has also been correlated with neurocognitive deficits (Cheung et al. 2000).
Of particular interest is a study published by Klein et al. (2002) in which the authors attempted to differentiate effects of tumor, radiotherapy, anticonvulsants, and surgical intervention in a group of 195 patients with low-grade glioma, 104 of whom had undergone radiotherapy. The group was compared to 100 patients with low-grade hematologic malignancies non-Hodgkin lymphoma and chronic lymphocytic leukemia (NHL/CLL) as well as 195 healthy controls. The use of anticonvulsants also had a significant negative impact on cognitive function. Glioma patients as a group had poorer cognitive functioning than both the NHL/CLL patients and the healthy controls. The use of radiation was correlated with cognitive deficits when irradiated glioma patients were compared with glioma patients who did not receive radiation. This effect was strongly dose- and fraction size-dependent. Patients who received >2 Gy/day of radiation accounted for nearly all cases of cognitive disability in this series. The authors concluded that tumor effects were responsible for the majority of cognitive deficits in low-grade glioma patients, although the delivery of radiation doses >2 Gy/day also had a significant impact on cognitive function (Klein et al. 2002).
4.1.3 Neurocognitive Decline in Patients with CNS Metastasis
Historically, brain radiation has been frequently cited as the major cause of neurocognitive decline in patients treated for metastases. One of the most misinterpreted studies on this subject is the Memorial Sloan-Kettering experience from DeAngelis et al. who reported an 11 % risk of radiation-induced dementia in patients undergoing WBRT for brain metastasis (DeAngelis et al. 1989a). Of the 47 patients who survived 1 year after WBRT, 5 patients (11 %) developed severe dementia. When these 5 patients are examined, all were treated in a fashion that would significantly increase the risk of late radiation toxicity (i.e., large daily fractions and concurrent radiosensitizer). Three patients received 5 and 6 Gy daily fractions, while a fourth patient received 6 Gy fractions with concurrent adriamycin. Only one patient received what is considered a standard radiation fractionation scheme (i.e., 30 Gy in 10 fractions), but this patient received a concurrent radiosensitizer (lonidamine). No patient who received the standard 30 Gy in 10 fractions WBRT alone experienced dementia. Even though the study included 232 patients in the initial analysis, it only examined the 47 patients who survived at least 1 year. The principles of conditional probability dictate that the 11 % risk is accurate only if a patient survives 1 year, which is significantly longer than most reported series. Therefore, a radiation-induced dementia risk of ≈2 % (5/232) might be a better estimate of the true probability ab initio for patients with brain metastasis treated with the various radiation doses and drugs used in that study.
Recent studies that have used sophisticated neurocognitive testing are clearly demonstrating that the brain tumor itself (presence, recurrence, and progression) has the greatest effect on neurocognitive decline. In the large phase III motexafin gadolinium study, the neurocognitive battery examined memory recall, memory recognition, delayed recall, verbal fluency, pegboard hand coordination, and executive function (Meyers et al. 2004). This study demonstrated that 21–65.1 % of patients had impaired functioning at baseline before treatment with WBRT (30 Gy in 10 daily fractions). Furthermore, patients who progressed in the brain after treatment experienced significantly worse scores in all of these individual tests.
Patients frequently present to the radiation oncologist already started on prophylactic anticonvulsants. This represents one of the most preventable causes of neurocognitive decline in brain tumor patients. Anticonvulsants are clearly known to adversely affect quality of life and neurocognition. In a study of 156 patients with low-grade glioma (85 % experiencing a seizure), Klein and colleagues correlated seizure-burden with quality of life and neurocognitive function (Klein et al. 2003). This study convincingly demonstrates the significant correlation between the increase in the number of anticonvulsants (even with lack of seizures) and the decrease in quality of life and neurocognitive function. Based on four negative randomized trials, the American Academy of Neurology recommends that prophylactic anticonvulsants not be initiated in newly diagnosed brain tumor patients who have not experienced a seizure (Glantz et al. 2000).
4.1.4 Radiation Necrosis
As described above, CNS radionecrosis is a focal, well-circumscribed lesion that develops in regions of the brain near the primary tumor that have received high doses of radiation. Necrosis is typically associated with focal neurologic deficits that correspond to the lesion’s location. Distinguishing these lesions from tumor recurrence can be problematic, but specialized imaging techniques may aid in diagnosis (Fig. 8). The lesion is frequently associated with significant cerebral edema, which precedes the development of radiation necrosis (Delattre et al. 1988). Edema and accompanying breakdown of the BBB increase parenchymal susceptibility to radiation necrosis by facilitating a cascade of local inflammatory mediators. This can be effectively inhibited with steroid therapy. Early (i.e., when focal edema is present but the area treated has not yet frankly necrosed) treatment with dexamethasone appears to improve outcomes significantly (Lee et al. 2002). If steroid therapy is delayed until the development of a cystic lesion, improvement of symptoms with medications alone is unlikely, and surgical excision of the mass may be indicated (Gutin 1991).
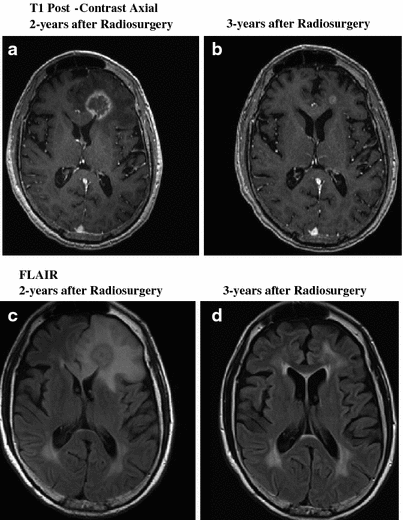

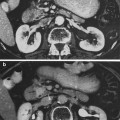
Fig. 8
Resolving Radiation Necrosis: the above images illustrate a case of proven radiation necrosis. The patient was a 62 years old female with 1.5 cm metastatic solitary brain metastasis from non-small cell lung cancer. She received 37.5 Gy in 2.5 Gy fractions of whole brain radiation, followed by radiosurgery boost to 24 Gy. Two years after radiosurgery boost, she developed radiation necrosis. Patient was almost asymptomatic; therefore, she was managed conservatively with no medical intervention. Over a course of 12 months, MRI slowly normalized. Images in the first row show T1 post-contrast axial images of a heterogeneously enhancing lesion with mass effect with subsequent resolution. (a 2-year, b 3-year) Lower images demonstrate FLAIR abnormalities indicating significant edema (c 2-year, d 3-year)
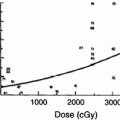
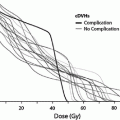
Necrosis develops months to years after RT (Sloan and Arnold 2003). Total dose and fraction size clearly predict for the development of radionecrosis (DeAngelis et al. 1989b; Sheline et al. 1980). Data from a large series (n = 1,032) of patients treated with RT for nasopharyngeal cancer show an increased risk of necrosis with fraction sizes ≥2 Gy/day (median total dose 62.5 Gy), twice-daily RT, shorter treatment times, and an increased value of the product of total dose and fraction size (Lee et al. 2002). The recent QUANTEC analysis summarized the incidence of necrosis following fractionated partial brain irradiation for patients receiving variable doses per fraction (Fig. 9) (Lawrence et al. 2010).
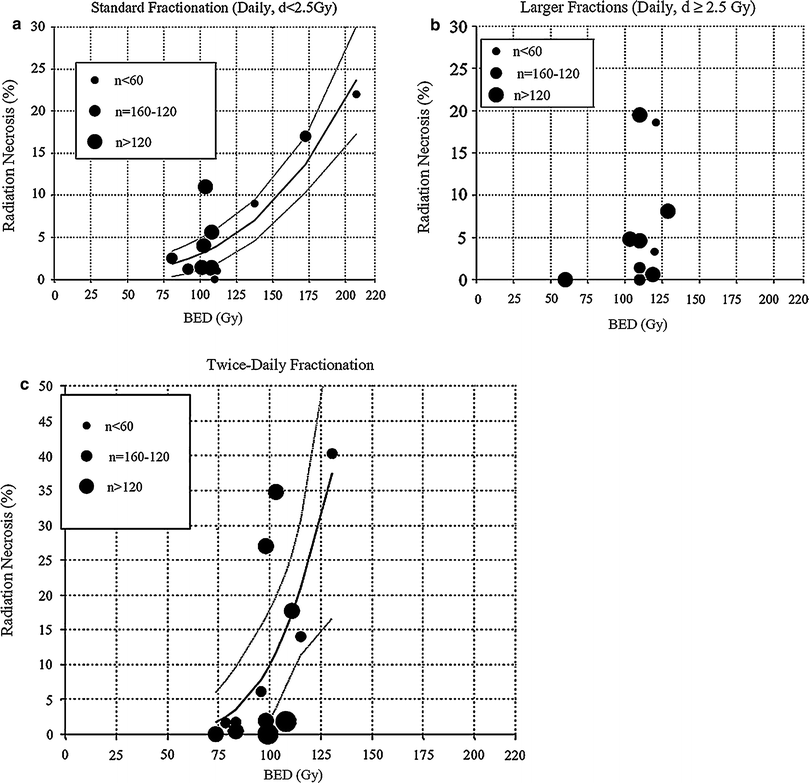

Fig. 9
Relationship between biologically effective dose (BED) and radiation necrosis after fractionated radiotherapy. Fit was done using nonlinear least-squares algorithm using Matlab software (The MathWorks, Natick, A). Nonlinear function chosen was probit model (similar functional form to Lyman model). Dotted lines represent 95 % confidence levels; each dot represents data from specific study, n = patient numbers as shown. a Fraction size <2.5 Gy, b
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree