UROGENITAL EMBRYOLOGY
Pronephros = forekidney
develops from mesoderm during 3rd week of gestation; involutes during 4th week of gestation → vestigial remnant / complete absence
Mesonephros = midkidney
develops during 4th week of gestation immediately caudal to pronephros, functions as interim kidney; degenerates around 8 weeks of gestation
(a) mesonephric tubules
→ paradidymis, epididymis, efferent ductules (M); epinephron (F)
(b) mesonephric (wolffian) duct
→ appendix epididymis, vas deferens, ejaculatory duct, seminal vesicles (M); vanishes (F)
[Caspar Friedrich Wolff (1733–1794), German anatomist, biologist, and embryologist in Berlin and St. Petersburg, cofounder of embryology, established doctrine of germ layers]
mnemonic: Gardener’s SEED
» female: Gartner duct, cyst
» male: Seminal vesicle
Epididymis
Ejaculatory duct
Ductus deferens
Paramesonephric (Müllerian) Duct
(grows alongside mesonephric duct)
[Johannes Peter Müller (1801–1858), German physiologist and comparative anatomist in Bonn and Berlin]
Male: degenerates ← production of müllerian inhibiting factor (MIF) by Sertoli cells of testis at about 6 weeks GA
→ prostatic utricle + appendix testis
Female: induced by wolffian duct at 5 weeks GA; grows caudally → joins in midline → fuses with outgrowth of urogenital sinus
→ uterus, fallopian tubes
Metanephros = hindkidney = permanent kidney
(1) metanephric diverticulum (ureteric bud) buds from mesonephric duct near its entry into the cloaca at 4th week; it lengthens + grows toward nephrogenic cord which becomes the metanephric blastema that divides and forms
→ ureter (mesonephric duct)
→ renal pelvis (first 4 dividing generations of duct)
→ calices (second 4 dividing generations of duct)
→ collecting tubules (10–12 generations of duct)
(2) metanephric blastema (= nephrogenic mesoderm) forms nephrons under the influence of ureteral bud → the end of collecting tubules induce clusters of metanephric blastema cells located at the periphery and along the sides of the medullary ray (= pyramid) except around the papilla
(3) metanephric vesicles form within clusters of metanephric blastema cells + elongate into S-shaped tubules which, by 12th week of gestation, result in
→ glomerulus
→ proximal convoluted tubule
→ loop of Henle
→ distal convoluted tubule
→ connective tissue
◊ Polycystic kidney disease is believed to be a failure of linkage!
Urogenital Sinus
forms from cloaca
→ develops into bladder + urethra (+ prostate)
Bladder
develops in 2nd–4th embryonal month
Urachus
= narrowed apex of fetal bladder continuous with allantoic stalk at the umbilicus
→ forms median umbilical ligament
(a) supravesical portion
(b) intramural portion
(c) intramucosal portion
SEX DEVELOPMENT
Indifferent Stage of Sexual Differentiation
Period: until 7th week of GA
Composition of undifferentiated gonad:
(1) Mesenchyme
condensation of mesenchyme forms genital ridges on both sides of midline between 6th thoracic and 2nd sacral segments; differentiates into interstitial (Leydig) cells within seminiferous tubules (= supporting stromal cells of testicular interstitium, including endothelial cells and vascular smooth muscle cells)
(2) Mesothelium
genital ridges are covered by proliferating mesothelium (coelomic epithelium); differentiates into Sertoli / supporting cells of the seminiferous tubules; forms tunica albuginea (vaginalis) as the serous covering of the testes
(3) Germ cells
form in wall of yolk sac and migrate along hindgut into genital ridge; differentiate into spermatogonia within seminiferous tubules; give rise to most testicular tumors
Chromosomes
› determine genotypic sex
› issue complex signaling pathways + hormones → modify primordial mesodermal cells → phenotypic sex
Formation of Testis
Period: around 8–12 weeks GA
• sex-determining factor localized on short arm of Y chromosome (SRY gene) → initiates male sex differentiation = seminiferous tubules
• Leydig cells secrete testosterone → stimulating growth of mesonephric (wolffian) structures
• Sertoli cells secrete müllerian-inhibiting factor → leading to regression of paramesonephric (müllerian) duct
Testicular Migration
| @ inguinal canal: | by 21 weeks |
| @ scrotum: | by 30 weeks at birth (in 97% of full-term infants); during first 3 months of life (most) |
Prenatal Evaluation of Sex
◊ Imaging of genitalia recommended only when
(a) medically indicated
(b) in twin gestation
Successful imaging of genitalia:
› in 60% by 14–18 weeks GA
› in 80–100% > 20 weeks GA
Errors in imaging of genitalia:
√ adducted fetal legs
√ US beam at wrong angle
Female:
√ 3 parallel lines between legs on transverse US
√ 3 bumps with clitoris in midline directed caudally
Male:
√ small semicircular structure (= scrotal sac)
√ penis in midline directed anteriorly and superiorly
√ visualization of testes in scrotum in late 2nd trimester
Ambiguous genitalia:
√ thorough fetal survey to detect associated anomalies
√ repeated US examinations with early GA
√ MRI with present oligohydramnios / multiple anomalies
RENAL ANATOMY
Adult Kidney
› forms by fusion of superior + inferior subkidneys (= metanephric lobes); the line of fusion runs obliquely forward and upward
√ separation of upper + lower groups of calices
√ indentation of cortical contour + echogenic line (= interrenicular septum = junctional parenchymal defect) delineates junctional parenchyma (often referred to as hypertrophic column of Bertin)
› consists of 20,000 lobules within 14 lobes (reniculi)
› initially located in pelvic region ventral to sacrum, ascending cranially at 9 weeks of gestation ← body growth caudal to kidneys + straightening of body curvature
› renal hilum at first ventrally located, eventually rotating medially by 90 degrees with renal ascent
Reniculus = renal lobe
= central core of medullary tissue enveloped by
(a) centrilobar cortex (= cortical arch) that covers the base of the pyramid → subsequently forming the renal cortex with loss of grooves
(b) mural cortex that wraps around sides of pyramid → and fuses with the mural cortex of adjacent lobe → to form renal septum (= column of Bertin)
› renal lobes completed by 28 weeks GA
√ ren lobatus (= interlobar surface grooves) present in fetus and infant, rare in adulthood
√ assimilation of independent lobes > 28 weeks GA makes renal surface smoother
› nephrogenesis completed by 36 weeks GA
Renal Size (in cm)
› < 1 year of age: 4.98 + 0.155 x age (in months)
› > 1 year of age: 6.79 + 0.22 x age (in years)
› adulthood: R kidney 10.74 ± 1.35 (SD); L kidney 11.10 ± 1.15 (SD)
› ratio of renal length (RL) to distance between first 4 lumbar transverse processes (4 TP) = 1.04 ± 0.22
Renal Echogenicity
A. ADULTHOOD
liver ≥ spleen ≥ renal cortex > renal medulla
B. INFANCY (in neonate up to 6 months of age)
√ cortex may be more echogenic than adjacent normal liver / spleen
Cause: glomeruli occupy 18% of cortex in neonate compared with 9% in adult
√ increase in corticomedullary differentiation
Cause: ratio of cortex to medulla 1.64÷1 in neonate compared with 2.59÷1 in adult
√ hypoechoic pyramids appear relatively large
Cause: thin immature cortex in neonate
N.B.: may be misinterpreted as dilated calices / renal cystic disease
√ renal sinus echogenicity less prominent
Cause: paucity of fat
Renal Vascular Anatomy
Hilar Arteries
Entry: renal hilum
1st order: main renal arteries at level of L1 / upper margin of L2
2nd order: 5 segmental branches = apical, anterior superior, anterior inferior, posterior, basilar
Extrahilar branching:
= branching of main renal artery prior to reaching hilum
Entry: renal hilum / direct as polar arteries
› early branching: within 1.5 cm from aorta
Resistive index: < 0.70 1 SD of several measurements = 0.04
Renal Polar Artery
Entry: without going through renal hilum directly into renal parenchyma at the poles
Types: superior polar artery; inferior polar artery (with supply of upper urinary tract)
Origin: main renal a., contralateral main renal a., aorta, iliac a., superior mesenteric a., inferior mesenteric a., celiac a., middle colic a., lumbar a., gonadal a., middle sacral a.
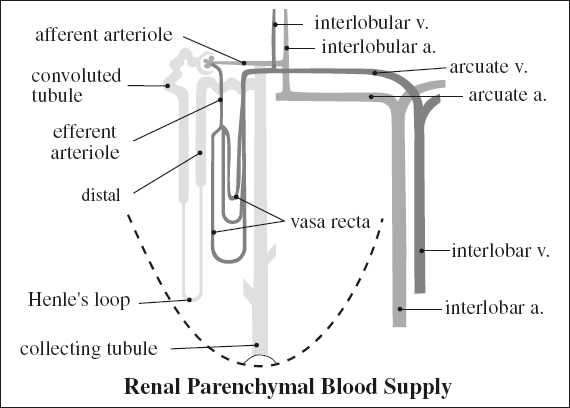
Capsular artery
= tiny vessels perfusing the renal capsule coursing tangentially to renal margin
Origin: main renal a., branch renal a., other retroperitoneal aa. (lumbar a.)
Anatomic Renal Artery Variants
Multiple renal arteries (25–30%):
unilaterally (32%); bilaterally (12%)
Number of renal arteries per kidney:
(a) one 71%
(b) two 24%
› 2 hilar arteries 12%
› 1 hilar + 1 superior polar artery 7%
› 1 hilar + 1 inferior polar artery 5%
(b) three 4%
(c) more than three 1%
◊ More than 2 renal arteries per kidney is a contraindication for donation! A small superior polar artery < 2 mm in diameter may be sacrificed.
Separate aortic ostium:
Main renal artery
= vessel with the greatest diameter
Accessory renal artery
= vessel with smaller diameter
= segmental artery originating from aorta / iliac a.
Supplementary artery:
Entry: renal hilum
Origin: aorta, iliac a., internal spermatic a., SMA, IMA, celiac trunk, middle colic a., lumbar a., middle sacral a., contralateral renal a.
Supply: lower pole (72%) > upper pole
Aberrant renal artery:
= segmental artery arising from superior mesenteric artery / internal spermatic artery
Renal Veins
Single right renal vein
without major extrarenal tributaries (85%)
with major extrarenal tributaries:
(a) right adrenal vein (30%)
(b) right gonadal vein (7%)
(c) lumbar + hemiazygos veins (3%)
Single preaortic left renal vein
without major extrarenal tributaries (86%)
with major extrarenal tributaries:
(a) left adrenal vein
(b) hemiazygos vein
(c) lumbar vein
(d) ascending lumbar vein
(e) left gonadal vein
Anatomic Renal Vein Variants
MULTIPLE RIGHT RENAL VEINS (15–30%)
(a) single right renal vein divides just before union with IVC 4%
(b) right gonadal vein joins the renal vein 6%
(c) accessory branch of adrenal vein enters right renal vein 31%
(d) lumbar / azygos vv. enter right renal vein 3%
CIRCUMAORTIC LEFT RENAL VEIN (5–6–17%)
SINGLE RETROAORTIC LEFT RENAL VEIN (2–3%)
LUMBAR VEINS JOINING LEFT RENAL VEIN (75%)
URETER
= retroperitoneal muscular conduit that carries urine from renal pelvis to urinary bladder
| Histo: | (a) | inner cell layer of watertight urothelium surrounded by |
| (b) | outer layer of smooth muscle → coordinated contraction |
Origin: ureteropelvic junction at intra / extrarenal pelvis
Terminus: ureterovesical junction
Length: ~ 25 cm
Diameter: < 3 mm
Congenital Ureteral Abnormalities
A. URETERAL DUPLICATION
1. Complete ureteral duplication
2. Partial ureteral duplication
B. SMOOTH MUSCLE DYSFUNCTION
1. UPJ obstruction
2. Primary congenital megaureter
C. ABNORMAL TERMINATION OF SINGLE URETER
1. Ectopic ureter
2. Ureterocele
3. Vesicoureteral reflux
Extrarenal Pelvis
= normal anatomic variant in which the renal pelvis lies predominantly outside of the renal sinus
√ may be dilated under normal circumstances
DDx: UPJ obstruction, distal obstruction
√ NO associated caliectasis
√ normal size of ureter
RETROPERITONEUM
= space posterior to peritoneal cavity extending from diaphragm to pelvic brim; separated from peritoneum anteriorly by posterior peritoneal fascia; bounded posteriorly by transversalis fascia
(a) anterior border: posterior peritoneal fascia (posterior to peritoneum)
(b) posterior border: transversalis fascia (attaches to psoas muscle)
Retroperitoneal Compartments
A. Anterior pararenal space
Boundaries: posterior parietal peritoneum (anteriorly), anterior renal fascia (posteriorly), lateroconal fascia (laterally)
→ superiorly joins with posterior renal fascia and attaches to crux of diaphragm
→ in the middle blends with connective tissues of central prevertebral space around great vessels
→ inferiorly joins with posterior renal fascia and attaches to great vessels
(a) pancreaticoduodenal space
Contents: pancreas, duodenum, root of small bowel mesentery
(b) pericolonic space
Contents: ascending colon + descending colon
B. Perirenal / perinephric space = capsula adiposa
Boundaries: fascia renalis = Gerota fascia = anterior (membrane of Toldt) + posterior (Zuckerkandl) renal fasciae
[Carl Toldt (1840–1920), Austrian anatomist, professor of anatomy in Prague and Vienna]
subdivided into multiple compartments by thin fibrous lamellae + incomplete bridging septa (Kunin septa) that attach to anterior + posterior renal fascia
→ forms superiorly closed inverted cone around adrenal gland + perirenal fat + upper half of kidney; fixed to diaphragmatic fascia above; abuts bare area of liver (R) + subphrenic space (L)
→ inferiorly forms cone around perirenal fat + lower pole of kidney blending with iliac fascia below
→ laterally closed by fusion of anterior + posterior sheath
→ medially open communicating with central prevertebral space
Contents: kidneys, adrenal glands, proximal renal collecting system, proximal ureters, perirenal fat, renal hilar vessels, lymphatic vessels
The perirenal fascia is not made up of distinct unilaminated fascia; rather, it is composed of multiple layers of variably fused embryonic mesentery, creating potential spaces between the retroperitoneal spaces.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree