CONGENITAL HEART DISEASE
Incidence of CHD in Liveborn Infants
Overall incidence: 8–9÷1000 livebirths
◊ Most common CHD: bicuspid aortic valve (2%) [usually not recognized before late infancy / childhood]
◊ ASD + VSD + PDA account for 45% of all CHD
◊ 12 lesions account for 89% of all CHD:
Ventricular septal defect 30.3%
Patent ductus arteriosus 8.6%
Pulmonic stenosis 7.4%
Ostium secundum defect 6.7%
Coarctation of aorta 5.7%
Aortic stenosis 5.2%
Tetralogy of Fallot 5.1%
Transposition 4.7%
Endocardial cushion defect 3.2%
Hypoplastic right ventricle 2.2%
Hypoplastic left heart syndrome 1.3%
TAPVR 1.1%
Truncus arteriosus 1.0%
Single ventricle 0.3%
Double outlet right ventricle 0.2%
High-risk pregnancy:
(1) Previous sibling with CHD: 2–5%
(2) Previous 2 siblings with CHD: 10–15%
(3) One parent with CHD: 2–10%
Congenital Abnormality of Aortic Valve
1. Bicuspid aortic valve
2. Unicuspid / unicomissural aortic valve
= single commissure in (commonly) L posterior position
Incidence: 0.02%
Cx: early aortic stenosis, aneurysmal dilatation of ascending aorta
3. Quadricuspid aortic valve
Incidence: 200 cases reported
Cx: regurgitation
4. Aneurysmal dilatation of sinus of Valsalva
| Cause: | (a) congenital: connective tissue disorder (Marfan), aortic insufficiency, bicuspid aortic valve, VSD |
(b) acquired: infection (endocarditis, syphilis, TB), medial degeneration, trauma, hypertension |
Congenital Abnormality of Mitral Valve
1. Endocardial cushion defect
2. Parachute mitral valve (Shone syndrome)
CHD Presenting in 1st Year of Life
1. VSD
2. d-Transposition of great vessels
3. Tetralogy of Fallot
4. Isolated coarctation
5. Patent ductus arteriosus
6. Hypoplastic left heart syndrome
Most common causes for CHF + PVH in neonate:
1. Left ventricular failure ← outflow obstruction
2. Obstruction of pulmonary venous return

CHD Compatible with Relatively Long Life
1. Mild tetralogy: mild pulmonic stenosis + small VSD
2. Valvular pulmonic stenosis: with relatively normal pulmonary circulation
3. Transposition of great vessels: some degree of pulmonic stenosis + large VSD
4. Truncus arteriosus: delicate balance between systemic + pulmonary circulation
5. Truncus arteriosus type IV: large systemic collaterals
6. Tricuspid atresia + transposition + pulmonic stenosis
7. Eisenmenger complex
8. Ebstein anomaly
9. Corrected transposition without intracardiac shunt
Classic Signs of Congenital Cardiovascular Anomalies
1. “Boot-shaped” heart
2. “Box-shaped” cardiomegaly
3. “Egg-on-a-string” sign
4. “Figure-of-3” and reverse “figure-of-3” sign
5. “Gooseneck” sign
6. “Scimitar” sign
7. “Snowman” sign
Juxtaposition of Atrial Appendages
1. Tricuspid atresia with transposition
2. Complete transposition
3. Corrected transposition of great arteries
4. DORV
Continuous Heart Murmur
1. PDA
2. AP window
3. Ruptured sinus of Valsalva aneurysm
4. Hemitruncus
5. Coronary arteriovenous fistula
Syndromes with CHD
5 p – (Cri-du-chat) Syndrome
Incidence of CHD: 20%
DiGeorge Syndrome
= congenital absence of thymus + parathyroid glands
1. Conotruncal malformation
2. Interrupted aortic arch
Down Syndrome
= MONGOLISM = TRISOMY 21
1. Endocardial cushion defect (25%)
2. Membranous VSD
3. Ostium primum ASD
4. AV communis
5. Cleft mitral valve
6. PDA
7. 11 rib pairs (25%)
8. Hypersegmented manubrium (90%)
Ellis-van Creveld Syndrome
Incidence of CHD: 50%
• polydactyly
√ single atrium
Holt-Oram Syndrome
= UPPER LIMB-CARDIAC SYNDROME
Incidence of CHD: 50%
1. ASD
2. VSD
3. Valvular pulmonary stenosis
4. Radial dysplasia
Hurler Syndrome
Cardiomyopathy
Ivemark Syndrome
Incidence of CHD: 100%
• asplenia
√ complex cardiac anomalies
Klippel-Feil Syndrome
Incidence of CHD: 5%
1. Atrial septal defect
2. Coarctation
Marfan Syndrome
= ARACHNODACTYLY
1. Annuloaortic ectasia
2. Aortic aneurysm
3. Aortic regurgitation
4. Pulmonary aneurysm
Noonan Syndrome
1. Pulmonary stenosis
2. ASD
3. Hypertrophic cardiomyopathy
Osteogenesis Imperfecta
1. Aortic valve insufficiency
2. Mitral valve insufficiency
3. Pulmonic valve insufficiency
Postrubella Syndrome
• low birth weight, deafness, cataracts, mental retardation
1. Peripheral pulmonic stenosis
2. Valvular pulmonic stenosis
3. Supravalvular aortic stenosis
4. PDA
Trisomy 13–15
VSD, tetralogy of Fallot, DORV
Trisomy 16–18
VSD, PDA, DORV
Turner Syndrome (XO)
= OVARIAN DYSGENESIS
Incidence of CHD: 35%
1. Coarctation of the aorta (in 15%)
2. Bicuspid aortic valve
3. Dissecting aneurysm of aorta
Williams Syndrome
= IDIOPATHIC HYPERCALCEMIA
• peculiar elfinlike facies, mental + physical retardation
• hypercalcemia (not in all patients)
1. Supravalvular aortic stenosis (33%)
2. ASD, VSD
3. Valvular + peripheral pulmonary artery stenosis
4. Aortic hypoplasia, stenoses of more peripheral arteries
SHUNT EVALUATION
Evaluation of L-to-R Shunts
A. AGE
› Infants:
(1) Isolated VSD
(2) VSD with CoA / PDA / AV canal
(3) PDA
(4) Ostium primum
› Children / adults:
(1) ASD
(2) Partial AV canal with competent mitral valve
(3) VSD / PDA with high pulmonary resistance
(4) PDA without murmur
B. SEX
99% chance for ASD / PDA in female patient
C. CHEST WALL ANALYSIS
√ 11 pair of ribs + hypersegmented manubrium → Down syndrome
√ pectus excavatum + straight back syndrome + funnel chest → prolapsing mitral valve
√ rib notching
D. CARDIAC SILHOUETTE
√ absent pulmonary trunk:
corrected transposition with VSD; pink tetralogy
√ left-sided ascending aorta:
corrected transposition with VSD
√ tortuous descending aorta:
aortic valve incompetence + ASD
√ huge heart:
persistent complete AV canal (PCAVC); VSD + PDA; VSD + mitral valve incompetence
√ enlarged left atrium:
intact atrial septum; mitral regurgitation (endocardial cushion defect, prolapsing mitral valve + ASD)

Shunt with Normal Left Atrium
A. Precardiac shunt
1. Anomalous pulmonary venous connection
B. Intracardiac shunt
1. ASD (8%)
2. VSD (25%)
C. Postcardiac shunt
1. PDA (12%)
Aortic Size in Shunts
A. Extracardiac shunts
√ aorta enlarged + hyperpulsatile
1. PDA
B. Pre- and intracardiac shunts
√ aorta small but not hypoplastic
1. Anomalous pulmonary venous return
2. ASD
3. VSD
4. Common AV canal
Left-to-Right Shunts Missed on Echocardiography
1. Sinus venosus defect
2. Patent ductus arteriosus
3. Anomalous pulmonary venous return
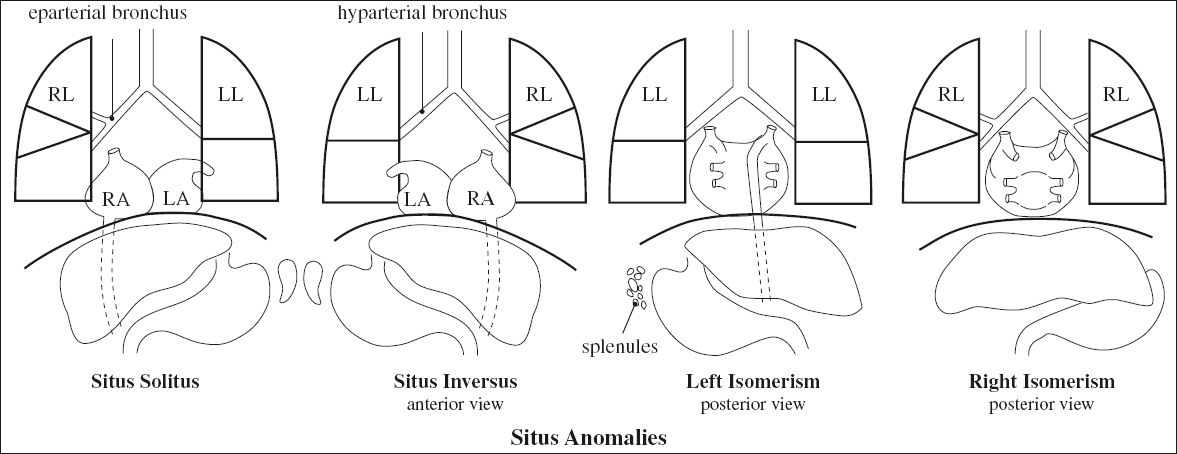
SITUS
= “position / site / location” referring to the position of the atria and viscera relative to the midline
Segmental Analysis of CHD
= 3-part Van Praagh notation of congenital heart disease corresponding to atria & ventricles & great vessels
◊ Only one type of situs anomaly is possible in any patient!
Step 1. Visceroatrial situs {X,_,_}:
solitus ={S}, inversus = {I}, ambiguus = {A}
(a) establish atrial situs by morphology of atrial appendages
√ broad blunt (triangular) appendage = RA
√ narrow pointed tubular (fingerlike) appendage = LA
◊ Morphologic RA is on patient’s right side
(b) establish atrial situs by lung morphology:
› morphologic RT lung
√ main RT lung bronchus is eparterial
› morphologic LT lung
√ main LT bronchus is hyparterial
The relationship between main RT + LT bronchi and pulmonary arteries is a reliable indicator of atrial arrangement.
(c) establish visceral situs:
√ largest lobe of liver on RT; stomach + spleen on LT
(b) establish thoracoabdominal situs:
√ supradiaphragmatic IVC connects to anatomic RA
√ RT lung + largest lobe of liver on right side
Supradiaphragmatic IVC / coronary sinus usually drain into anatomic RA (= rule of venoatrial concordance)
◊ Atrial + thoracoabdominal situs are usually concordant!
Step 2. Ventricular loop orientation {_,X,_}:
Embryology:
components of craniocaudal tube are truncus arteriosus (future great vessels), bulbus cordis (future right ventricle), embryonic LV, primitive atria, sinus venosus; tube curves toward right (D-loop) placing RV to right of LV
(a) identify morphologic RV and LV
(b) determine location of RV relative to LV
Hand rule: approaching heart from anteriorly place right / left hand in RV with palm against IVS. If right thumb is in inflow tract + right fingers in outflow tract = D-loop
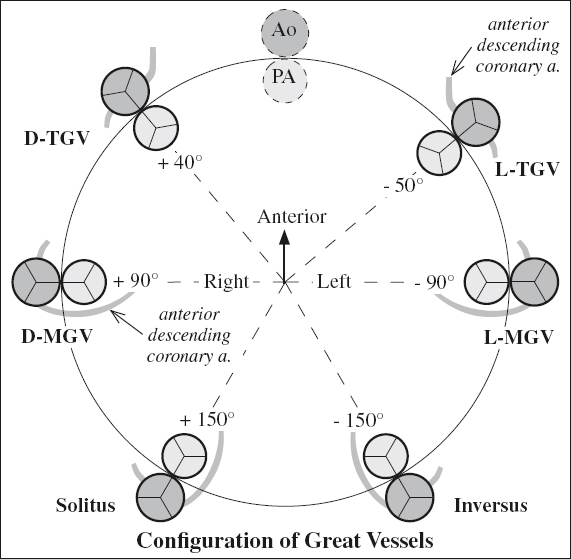
Step 3. Position of great vessels {_,_,X}:
(a) identify morphologic aorta (Ao supplies at least 1 coronary artery + most systemic arteries) and main pulmonary artery (MPA supplies right + left pulmonary artery)
(a) designate position of Ao relative to MPA at level of aortic + pulmonic valves:
› normal or inverse position: solitus ={S}, inversus = {I}
› transposition of great arteries: dextrotransposition = {D-TGV}, levotransposition = {L-TGV}
› malposition if both arteries override / originate from same ventricle: Ao rightward from MPA = {D-MGV}, Ao leftward from MPA = {L-MGV}
Situs Solitus = Normal / Usual Situs

Associated with:
| (a) levocardia | : | 0.6–0.8% chance for CHD |
| (b) dextrocardia | : | 95% chance for CHD |
SITUS ANOMALY
Associated with: CHD, primary ciliary dyskinesia, abdominal anomalies (eg, intestinal malrotation)
Situs Inversus
= mirror-image arrangement of situs solitus
Frequency: 0.01%Associated with:
(a) dextrocardia = situs inversus totalis (usual variant): 3–5% chance for CHD (eg, Kartagener syndrome in 20%)
(b) levocardia (extremely rare): 95% chance for CHD
Situs Ambiguus = Heterotaxy
[heteros, Greek = other / different; taxis, Greek = arrangement]
= visceral malposition + dysmorphism associated with indeterminate atrial arrangement
Associated with: CHD in 50–100%
Subclassification:
1. Asplenia syndrome = double / bilateral right-sidedness
2. Polysplenia syndrome = double / bilateral left-sidedness
Bronchial Situs
Classification:
1. Right bronchial isomerism
√ bilateral eparterial upper lobe bronchi = morphologically right lung
2. Left bronchial isomerism
√ bilateral hyparterial upper lobe bronchi = morphologically left lung
The relationship of the upper lobe bronchus to the ipsilateral pulmonary artery is the most reliable marker of bronchial situs at CT for details see Anatomy and Function of Lung
Less reliable: number of lobes, ratio of left to right main bronchial lengths, bronchial branching pattern
Cardiac Malposition
= location of heart other than within left hemothorax in situs solitus or within left hemithorax when other organs are ambiguous
◊ Determined by base-apex axis; no assumption is made regarding cardiac chamber / vessel arrangement
In general, cardiac and situs anomalies are less frequently found with a concordant position of the cardiac apex, stomach, and aortic arch.
A. POSITION OF CARDIAC APEX
1. Levocardia = apex directed leftward
2. Dextrocardia = apex directed rightward
3. Mesocardia = vertical / midline heart (usually with situs solitus)
√ atrial septum characteristically bowed into LA in cardiac situs solitus with dextrocardia + cardiac situs inversus with levocardia (DDx: juxtapositioned atrial appendages)

◊ Does not imply a particular internal structure / specific location of other thoracoabdominal organs!
B. CARDIAC DISPLACEMENT
by extracardiac factors (eg, lung hypoplasia, pulmonary mass)
1. Dextroposition
suggests hypoplasia of ipsilateral pulmonary artery (PAPVR implies scimitar syndrome)
2. Levoposition
3. Mesoposition
C. CARDIAC INVERSION
= alteration of normal relationship of chambers
1. D-bulboventricular loop
2. L-bulboventricular loop
D. TRANSPOSITION
= alteration of anterior-posterior relationship of great vessels
CYANOTIC HEART DISEASE
| Chemical cyanosis | = | PaO2 ≤ 94% |
| Clinical cyanosis | = | PaO2 ≤ 85% |
◊ Decrease in hemoglobin delays detectability!
Most common cause of cyanosis:
| › | in newborn: | transposition of great vessels |
| › | in child: | tetralogy of Fallot! |
N.B.: tricuspid atresia = the great mimicker
Increased Pulmonary Blood Flow with Cyanosis
= ADMIXTURE LESIONS
= bidirectional shunt with 2 components:
(a) mixing of saturated blood (L-R shunt) and unsaturated blood (R-L shunt)
(b) NO obstruction to pulmonary blood flow
Evaluation process:
√ cardiomegaly
√ increased pulmonary blood flow
√ concave main pulmonary artery:
√ PA segment absent = transposition
√ PA segment present:
(a) L atrium normal (= extracardiac shunt) = TAPVR
(b) L atrium enlarged (= intracardiac shunt) = truncus arteriosus
N.B.: Overcirculation + cyanosis = complete transposition until proven otherwise!
Admixture Lesions = T-lesions
mnemonic: 5 T’s + CAD
Transposition of great vessels = complete TGV ± VSD
◊ Most common cause for cyanosis in neonate
Tricuspid atresia with or without transposition + VSD
◊ 2nd most common cause for cyanosis in neonate
Truncus arteriosus
Total anomalous pulmonary venous return (TAPVR) above diaphragm:
(a) supracardiac
(b) cardiac (coronary sinus / right atrium)
“Tingle” = single ventricle
Common atrium
Aortic atresia
Double-outlet right ventricle (DORV type I) / Taussig-Bing anomaly (DORV type II)
Clues:
√ skeletal anomalies: Ellis-van Creveld syndrome (truncus / common atrium)
√ polysplenia: common atrium
√ R aortic arch: persistent truncus arteriosus
√ ductus infundibulum: aortic atresia
√ pulmonary trunk seen: supracardiac TAPVR; DORV; tricuspid atresia; common atrium
√ ascending aorta with leftward convexity: single ventricle
√ dilated azygos vein: common atrium + polysplenia + interrupted IVC; TAPVR to azygos vein
√ left-sided SVC: vertical vein of TAPVR
√ “waterfall” right hilum: single ventricle + transposition
√ large left atrium (rules out TAPVR)
√ prominent L heart border: single ventricle with inverted rudimentary RV; levoposition of appendage of RA (tricuspid atresia + transposition)
√ age of onset ≤ 2 days: aortic atresia
Decreased Pulmonary Blood Flow with Cyanosis
= two components of
(a) impedance of blood flow through right heart ← obstruction / atresia at pulmonary valve / infundibulum
(b) R-to-L shunt
• pulmonary circulation maintained through systemic arteries / PDA
√ normal / decreased pulmonary blood flow
√ concave main pulmonary artery
√ cardiomegaly
√ restrictive intracardiac R-to-L shunt
mnemonic: P2 TETT
Pulmonic stenosis with ASD
Pulmonic atresia
Tetralogy of Fallot
Ebstein anomaly
Tricuspid atresia with pulmonic stenosis
Transposition of great vessels with pulmonic stenosis
A. SHUNT AT VENTRICULAR LEVEL
1. Tetralogy of Fallot
2. Tetralogy physiology (associated with pulmonary obstruction):
› complete / corrected transposition
› single ventricle
› DORV
› tricuspid atresia (PS in 75%)
› asplenia syndrome
√ prominent aorta with L / R aortic arch; inapparent pulmonary trunk
√ normal RA (without tricuspid regurgitation)
√ normal-sized heart ← escape mechanism into aorta
Clues:
1. Skeletal anomaly (eg, scoliosis): tetralogy (90%)
2. Hepatic symmetry: asplenia
3. Right aortic arch: tetralogy, complete transposition, tricuspid atresia
4. Aberrant right subclavian artery: tetralogy
5. Leftward convexity of ascending aorta: single ventricle with inverted right rudimentary ventricle, corrected transposition, asplenia, JAA (tricuspid valve atresia)
B. SHUNT AT ATRIAL LEVEL
mnemonic: PET
1. Pulmonary stenosis / atresia with intact ventricular septum
2. Ebstein malformation + Uhl anomaly
3. Tricuspid atresia (ASD in 100%)
√ moderate to severe cardiomegaly
√ RA dilatation
√ RV enlargement ← massive tricuspid incompetence
√ inapparent aorta
√ left aortic arch
Pulmonary Venous Hypertension with Cyanosis
(a) during 1st week of life
1. Hypoplastic left heart syndrome
√ marked cardiomegaly
2. TAPVR below diaphragm
√ normal cardiac size
(b) during 2nd week of life
3. Aortic coarctation
4. Aortic atresia
(c) during 4th–6th week of life
5. Critical aortic stenosis
6. Endocardial fibroelastosis
7. Anomalous origin of LCA
8. Atresia of common pulmonary vein
ACYANOTIC HEART DISEASE
Increased Pulmonary Blood Flow without Cyanosis
= indicates L-R shunt with increased pulmonary blood flow (shunt volume > 40%)
A. WITH LEFT ATRIAL ENLARGEMENT
indicates shunt distal to mitral valve = increased volume without escape defect
1. VSD (25%): small aorta in intracardiac shunt
2. PDA (12%): aorta + pulmonary artery of equal size in extracardiac shunt
3. Ruptured sinus of Valsalva aneurysm (rare)
4. Coronary arteriovenous fistula (very rare)
5. Aortopulmonary window (extremely rare)
B. WITH NORMAL LEFT ATRIUM indicates shunt proximal to mitral valve = volume increased with escape mechanism through defect
1. ASD (8%)
2. Partial anomalous pulmonary venous return (PAPVR) + sinus venosus ASD
3. Endocardial cushion defect (ECD) (4%)
Normal Pulmonary Blood Flow without Cyanosis
A. OBSTRUCTIVE LESION
1. Right ventricular outflow obstruction
(a) at level of pulmonary valve: subvalvular / valvular / supravalvular pulmonic stenosis
(b) at level of peripheral pulmonary arteries:
peripheral pulmonary stenosis
2. Left ventricular inflow obstruction
(a) at level of peripheral pulmonary veins:
pulmonary vein stenosis / atresia
(b) at level of left atrium: cor triatriatum
(c) at level of mitral valve:
supravalvular mitral stenosis, congenital mitral stenosis / atresia, “parachute” mitral valve
3. Left ventricular outflow obstruction
(a) at level of aortic valve:
anatomic subaortic stenosis, functional subaortic stenosis (IHSS), valvular aortic stenosis, hypoplastic left heart, supravalvular aortic stenosis
(b) at level of aorta:
interruption of aortic arch, coarctation of aorta
B. CARDIOMYOPATHY
1. Endocardial fibroelastosis
2. Hypertrophic cardiomyopathy
3. Glycogen storage disease
C. HYPERDYNAMIC STATE
1. Noncardiac AVM: cerebral AVM, vein of Galen aneurysm, large pulmonary AVM, hemangioendothelioma of liver
2. Thyrotoxicosis
3. Anemia
4. Pregnancy
D. MYOCARDIAL ISCHEMIA
1. Anomalous left coronary artery
2. Coronary artery disease (CAD)
ACQUIRED HEART DISEASE
√ LA enlargement = MV disease
√ dilated ascending aorta = aortic valve disease
√ RA enlargement = tricuspid valve disease
Pressure Overload
1. Systemic hypertension
2. Aortic stenosis
3. Mitral stenosis
Decreased Compliance
1. Myocardial infarction
2. Hypertrophic cardiomyopathy
3. Restrictive cardiomyopathy
Volume Overload
1. Aortic insufficiency
2. Mitral insufficiency
3. Tricuspid insufficiency
Systolic Anterior Motion of Mitral Valve
1. Hypertrophic Cardiomyopathy
2. Hypertensive heart
3. Diabetes mellitus
4. Acute myocardial infarction
5. Mitral valve dysfunction / repair
Concentric Left Ventricular Hypertrophy
1. Symmetric hypertrophic cardiomyopathy

2. Amyloidosis
3. Cardiac sarcoidosis
4. Athlete’s heart
√ ↑ LV mass, ↑ LV diastolic cavity dimension, ↑ LV wall thickness
√ lack of areas of delayed myocardial hyperenhancement
Dx (80% sensitive, 99% specific):
LV Thicknessdiastole ÷ Volumeend-diastole (corrected to body surface area) < 0.15 mm/m2/mL
5. Fabry disease
= rare X-linked autosomal recessive metabolic storage disorder caused by lysosomal a-galactosidase
√ concentric thickening of LV wall
√ delayed hyperenhancement of LV midwall on CEMR
6. Adaptive LV hypertrophy: hypertension, aortic stenosis
CARDIOMEGALY
1. CHF
2. Multivalvular disease
3. Pericardial effusion
√ absence of pulmonary venous hypertension + hydrostatic edema
Cardiothoracic Ratio
= widest transverse cardiac diameter ÷ widest inside thoracic diameter
= primarily a measure of LV dilatation
| < 0.45 | normal |
| 0.45–0.55 | mild cardiomegaly |
| > 0.55 | moderate / severe cardiomegaly |
Falsely normal: with LV enlargement in up to 66%, moderate enlargement of LA + RV
Falsely elevated: in expiration, in recumbent position
Vascular Pedicle Width
= distance on a horizontal line between (1) point where right mainstem bronchus + SVC cross and (2) point where left subclavian artery crosses horizontal line
| 48 ± 5 mm | normal |
| > 53 mm | in 60% of cardiogenic edema, in 85% of volume overload |
Cardiomegaly in Newborn
A. NONCARDIOGENIC
(a) metabolic:
1. Ion imbalance in serum levels of sodium, potassium, and calcium
2. Hypoglycemia
(b) decreased ventilation
1. Asphyxia
2. Transient tachypnea
3. Perinatal brain damage
(c) erythrocyte function
1. Anemia
2. Erythrocythemia
(d) endocrine
1. Glycogen storage disease
2. Thyroid disease: hypo- / hyperthyroidism
(e) infant of diabetic mother
(f) arteriovenous fistula
1. Vein of Galen aneurysm
2. Hepatic angioma
3. Chorioangioma
B. CARDIOGENIC
1. Arrhythmia
2. Myo- / pericarditis
4. Myocardial infarction
5. Congenital heart disease
Abnormal Heart Chamber Dimensions
A. LEFT VENTRICULAR VOLUME OVERLOAD
1. VSD
2. PDA
3. Mitral incompetence
4. Aortic incompetence
B. LEFT VENTRICULAR HYPERTROPHY
1. Coarctation
2. Aortic stenosis
C. RIGHT VENTRICULAR VOLUME OVERLOAD
1. ASD
2. Partial APVR / total APVR
3. Tricuspid insufficiency
4. Pulmonary insufficiency
5. Congenital / acquired absence of pericardium
[6. Ebstein anomaly] – not truly RV
D. RIGHT VENTRICULAR HYPERTROPHY
1. Pulmonary valve stenosis
2. Pulmonary hypertension
3. Tetralogy of Fallot
4. VSD
E. Fixed subvalvular aortic stenosis
F. Hypoplastic left / right ventricle, common ventricle
G. Congestive cardiomyopathy
Right Atrial Enlargement
Cause: tricuspid stenosis / regurgitation, ASD, atrial fibrillation, dilated cardiomyopathy, Ebstein anomaly, pulmonary atresia
◊ RA enlarges in rightward + posterior direction
PA CXR:
√ prominent round superior border at junction with SVC
√ > 5.5 cm from midline to most lateral RA margin
√ > 2.5 cm from right vertebral margin
√ > 50% vertical height of RA compared with cardiovascular mediastinal height (from top of aortic arch to base of heart)
LAT CXR:
√ sharp horizontal interface with lung above RV
√ displacement of heart posterior to IVC mimicking LV enlargement
Right Ventricular Enlargement
Cause: pulmonary valve stenosis, cor pulmonale, ASD, tricuspid regurgitation, dilated cardiomyopathy, secondary to LV failure
◊ RV enlarges in anterior, superior + leftward direction causing levorotation of heart
PA CXR:
◊ Only extreme dilatation causes recognizable signs on frontal view!
√ straightening / convexity of left upper cardiac contour
√ upturned cardiac apex
√ left upper cardiac margin parallels left mainstem bronchus as a long convex curvature
√ increased distance between left upper cardiac margin + left mainstem bronchus
√ small appearance of rotated aortic arch + SVC
√ large appearance of main pulmonary artery
LAT CXR (most sensitive view):
√ filling of retrosternal air space by prominent convexity of anterior heart border > ⅓ of distance from sternodiaphragmatic angle to the point where trachea meets sternum
Left Atrial Enlargement
Cause:
(a) acquired: mitral stenosis / regurgitation, LV failure, LA myxoma
(b) congenital: VSD, PDA, hypoplastic left heart
◊ LA enlarges in multiple directions
PA CXR:
√ right retrocardiac double density with inferomedial curvature (earliest sign)
√ > 7.0 (female) / 7.5 (male) cm distance between midpoint of undersurface of left mainstem bronchus + right lateral LA shadow
√ left retrocardiac double density
√ > 75° splaying of carina with horizontal orientation of distal left mainstem bronchus
√ enlarged left-convex left atrial appendage ± calcifications (← rheumatic heart disease in 90%)
LAT CXR:
√ increased convexity of posterosuperior cardiac margin
√ posterosuperior atrial convexity crosses vertical plane formed by tracheal midline + upper lobe bronchus
√ posterior displacement of barium-filled esophagus
√ posterior displacement of LUL bronchus
Left Ventricular Enlargement
Cause:
(a) pressure overload: hypertension, aortic stenosis
(b) volume overload: aortic or mitral regurgitation, VSD
(c) wall abnormalities: LV aneurysm, hypertrophic cardiomyopathy
◊ LV enlarges in posterior, inferior + leftward direction
PA CXR:
√ leftward displacement of downturned cardiac apex = left ventricular configuration
√ depression of left hemidiaphragm + gastric bubble (with diaphragmatic inversion)
LAT CXR:
√ increased convexity of posteroinferior cardiac margin
√ posterior cardiac margin projects > 1.8 cm posterior to IVC measured at a point 2 cm above intersection of IVC with right hemidiaphragm (Hofman-Rigler rule)
Flattening / Inversion of Interventricular Septum
= position determined by pressure difference between RV + LV
√ normally convex shape with slight right-sided bulge during ventricular filling (LV diastolic pressure > RV pressure)
(a) RV volume / pressure overload:
1. ASD
2. Acute / chronic cor pulmonale
(b) pericardial abnormality:
1. Cardiac tamponade (↑ pericardial pressure)
2. Constrictive pericarditis (↓ pericardial compliance)
Enlargement of Coronary Sinus
Conditions Causing RA Dilatation
1. Tricuspid stenosis / regurgitation
2. Right ventricular dysfunction (failure): CHF, cardiomyopathy, atrial fibrillation, atrioventricular node reentrant tachycardia
3. Severe pulmonary hypertension
4. Right atrial hypertension
5. Cardiac tamponade
Congenital Structural Anomaly
(a) increased volume of systemic venous blood
(1) persistent left SVC draining into coronary sinus
(2) interrupted IVC with hemiazygos continuation to a left SVC / hepatic veins connecting directly into coronary sinus
The coronary sinus dilates most commonly due to a left SVC draining into the coronary sinus!
(b) L-to-R shunt: oxygenated blood
› low-pressure shunt:
1. Interatrial shunt (= coronary sinus-ASD) LA → unroofed coronary sinus
An unroofed coronary sinus is a complete / partial wall defect partitioning the coronary sinus from the left atrium!
2. TAPVR
› high-pressure shunt:
3. Coronary arteriovenous fistula
Small Coronary Sinus
A. ACQUIRED
1. Atrial systole
2. Lipomatosis
3. Small heart
4. Stenosis
B. CONGENITAL
1. Atresia
2. Hypoplasia: corrected TGA
3. Unroofed coronary sinus
4. CHD: corrected TGA, tetralogy of Fallot, Ebstein
Neonatal Cardiac Failure
A. LEFT-SIDED OBSTRUCTIVE LESIONS
1. Segmental hypoplasia of aorta
2. Critical coarctation of the aorta
3. Aortic valve stenosis
4. Asymmetrical septal hypertrophy / hypertrophic obstructive cardiomyopathy
5. Mitral valve stenosis
6. Cor triatriatum
B. VOLUME OVERLOAD
1. Congenital mitral valve incompetence
2. Corrected transposition with left (= tricuspid) AV valve incompetence
3. Congenital tricuspid insufficiency
4. Ostium primum ASD
C. MYOCARDIAL DYSFUNCTION / ISCHEMIA
1. Nonobstructive cardiomyopathy
2. Anomalous origin of LCA from pulmonary trunk
3. Primary endocardial fibroelastosis
4. Glycogen storage disease (Pompe disease)
5. Myocarditis
D. NONCARDIAC LESIONS
1. AV fistulas: hemangioendothelioma of liver, AV fistula of brain, vein of Galen aneurysm, large pulmonary AV fistula
2. Transient tachypnea of the newborn
3. Intraventricular / subarachnoid hemorrhage
4. Neonatal hypoglycemia (low birth weight, infants of diabetic mothers)
5. Thyrotoxicosis (transplacental passage of LATS hormone)
Congestive Heart Failure & Cardiomegaly
mnemonic: Ma McCae & Co.
Myocardial infarction
anemia
Malformation
cardiomyopathy
Coronary artery disease
aortic insufficiency
effusion
Coarctation
Congenital Cardiomyopathy
mnemonic: CAVE GI
Cystic medial necrosis of coronary arteries
Aberrant left coronary artery / Absent coronary artery
Viral myocarditis
Endocardial fibroelastosis
Glycogen storage disease (Pompe)
Infant of diabetic mother / Ischemia
Delayed Myocardial Hyperenhancement on MRI
A. Ischemic heart disease (MI)
B. Nonischemic
1. Dilated / hypertrophic cardiomyopathy
2. Myocarditis
3. Sarcoidosis
4. Systemic sclerosis
5. Endocardial fibroelastosis
6. Fabry disease
7. Chagas disease
8. RV pressure overload
9. Amyloidosis
10. Cardiac transplantation
11. Uremia
Subendocardial Enhancement
1. Ischemic heart disease
2. Amyloidosis
3. Löffler endocarditis (hypereosinophilic syndrome)
Transmural Enhancement
1. Ischemic heart disease
2. Myocarditis
Subepicardial Enhancement
1. Myocarditis
Mesocardial Enhancement
= delayed enhancement in midinterventricular septum
1. Hypertrophic cardiomyopathy
2. Dilated cardiomyopathy
Nodular Patchy Enhancement
1. Amyloidosis
2. Myocarditis
3. Cardiac sarcoidosis
Cardiac Accumulation of FDG
Myocardial Uptake
1. Physiologic FDG uptake ← depending on levels of blood glucose and free fatty acids
√ none / diffuse / focal uptake in walls of ventricles
Remedy: uptake suppressed by low-carbohydrate dinner followed by 12-hour fast
2. Drug interaction
(a) lowering uptake:
› Bezafibrate for treating hyperlipidemia
› Levothyroxine (thyroid hormone)
(b) increasing uptake:
› benzodiazepines: Diazepam
3. Sarcoidosis
√ hypermetabolic myocardial foci in nonperivascular distribution
4. Biventricular chemotherapy-induced cardiomyopathy
√ increased metabolic activity in ventricular myocardium
5. Ventricular enlargement associated with systemic + pulmonary hypertension and valvular heart disease
√ diffuse uniform increase in myocardial activity
6. Inflammatory myocarditis
7. Endocarditis ← infected valve
8. Neoplasia: sarcoma, metastasis (breast, lung, melanoma, lymphoma), myxoma
9. Coronary artery disease ← shift from fatty acid metabolism to glucose utilization ← ischemia
ATRIAL UPTAKE
1. Atrial fibrillation
2. Radiofrequency ablation for atrial fibrillation
3. Crista terminalis: ? physiologic
Pericardial Uptake
1. Post–radiation pericarditis
2. Malignant pericardial effusion
3. Epipericarditis
4. Lipomatous hypertrophy interatrial septum
CARDIAC TUMOR
◊ Extremely rare; often asymptomatic until very large!
• symptoms of cardiopulmonary diseases:
• congestive heart failure: dyspnea, orthopnea, peripheral edema, paroxysmal nocturnal dyspnea
• palpitations, heart murmur, cough, chest pain
• symptoms caused by peripheral emboli to cerebral / systemic / coronary circulation: syncope
• weight loss, fever, malaise
Location: intracavitary (obstruction, emboli), intramural (arrhythmia), pericardial (tamponade)
CXR:
√ cardiomegaly, pericardial effusion
√ signs of CHF
√ abnormal cardiac contour
√ pleural effusion
DDx: thrombus, pericardial and bronchogenic cyst, intrathoracic neoplasm, gastrointestinal hernia
Primary Heart Tumor
Prevalence: 0.002–0.250%; 0.001–0.030% (autopsy series)
Benign Heart Tumor in Adults (75%)
◊ More common than malignant neoplasms
1. Myxoma (with almost 50% the most common primary cardiac tumor; 25% of all cardiac tumors)
2. Papillary fibroelastoma (10% of all primary cardiac tumors; with 75% most common valvular tumor)
3. Lipoma (10% of all cardiac tumors)
4. Hydatid cyst (uncommon):
√ localized bulge of left cardiac contour
√ curvilinear / spotty calcifications (resembling myocardial aneurysm)
Cx: may rupture into cardiac chamber / pericardium
Malignant Heart Tumors (25%)
Prevalence: 25% of all cardiac tumors in adults;
10% of all cardiac tumors in children
1. Sarcomas
2. Rhabdomyosarcoma
3. Lymphoma (rare)
4. Pericardial mesothelioma
5. Malignant teratoma
6. Multiple cardiac myxomas
Secondary (Metastatic) Heart Tumor
◊ Most common cause of a neoplastic cardiac mass!
Age: typically > 60 years with disseminated tumor
Prevalence: metastatic÷primary disease = 30÷1; 10–12% of cancer patients develop metastases to the heart (autopsy)
Origin: lung, breast, esophagus, melanoma, lymphoma, leukemia
◊ Melanoma has the highest prevalence of cardiac seeding of any neoplasm!
• asymptomatic (frequently)
• arrhythmia (most common clinical sign)
• chest wall pain ← transient ischemic attack
• CHF (shortness of breath, fatigue, peripheral edema)
Site:
(a) peri- / epicardium (most common): lung > breast > lymphoma > leukemia > breast > esophagus
(b) myocardium: malignant melanoma; secondary extension to myocardium from epicardium
◊ Most common sites for myocardial lesions are LV free wall + interventricular septum (= greatest myocardial mass)
(c) endocardial / intracavitary (in only 5%)
(d) transvenous extension: RCC, hepatoma, adrenal adenocarcinoma
√ nonspecific imaging features
ECHO: iso- / hyperechoic
US/CT/MR: enhancement
MR: T1-hypointense + T2-hyperintense
Exception: T1-hyperintensity of melanoma, hemorrhagic lesion
Prognosis: death (⅓) ← cardiac tamponade / CHF / coronary artery invasion, sinoatrial node invasion
Cavoatrial Tumor Extension
= neoplasm arising in infradiaphragmatic sites
1. Hepatocellular carcinoma
extension into hepatic veins / IVC (7.5%), into RA (4%)
2. RCC
invasion of renal vein / IVC (5–15%), into RA (1%)
4. Wilms tumor
into renal veins / IVC (4–10%), into RA (1%)
5. Adrenocortical carcinoma
6. Uterine leiomyoma
7. Leiomyosarcoma of IVC
8. Osteogenic sarcoma of pelvis
N.B.: sudden death may occur when tumor in RA intermittently obstructs tricuspid valve leading to low cardiac output
Congenital Cardiac Tumor
The majority of primary cardiac tumors in children are benign, rhabdomyoma and fibroma accounting for 70%!
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree