AMYLOIDOSIS
= extracellular deposits of insoluble fibrillar protein (most commonly amyloid AL = light chain) in myocardial interstitium
Incidence: AL amyloidosis (50%); AA amyloidosis (2%);
isolated myocardial amyloid deposition (in 25% of autopsied patients > 85 years of age)
Path: commonly involvement of all 4 chambers
• asymptomatic / fatigue, weakness
• angina, CHF (diastolic dysfunction ⇉ restrictive cardiomyopathy), arrhythmia
CXR:
√ normal / generalized cardiomegaly
√ pulmonary congestion
√ pulmonary deposits of amyloid
NUC:
√ striking uptake of 99mTc-pyrophosphate greater than bone (50–90%)
ECHO:
√ granular sparkling appearance of myocardium
√ LV wall thickening
√ decreased LV systolic + diastolic function
MR:
√ diffuse decrease in SI on T1WI
√ diffuse pattern of ventricular myocardial hypertrophy
√ increase in thickness of interatrial septum + right atrial free wall > 6 mm (SPECIFIC)
[DDx: ischemic heart disease thins myocardium]
CEMR:
√ predominant late enhancement of entire subendocardial circumference (HIGHLY SPECIFIC + SENSITIVE)
Dx: endomyocardial Bx with potential for severe complications
ANOMALOUS (INNOMINATE) BRACHIOCEPHALIC ARTERY COMPRESSION SYNDROME
= origin of R innominate (brachiocephalic) artery to the left of trachea coursing to the right
√ anterior tracheal compression
• ablation of right radial pulse by rigid endoscopic pressure
√ posterior tracheal displacement
√ focal collapse of trachea at fluoroscopy
√ pulsatile indentation of anterior tracheal wall by innominate artery on MR
Rx: surgical attachment of innominate artery to manubrium
ANOMALOUS LEFT CORONARY ARTERY
= A NOMALOUS ORIGIN OF L EFT C ORONARY A RTERY FROM P ULMONARY A RTERY SYNDROME = ALCAPA = BLAND-WHITE-GARLAND SYNDROME
Prevalence: 1÷300,000 live births; 0.25–0.50% of congenital heart defects
◊ One of the most common causes of myocardial ischemia + infarction in children!
Age: infancy / early childhood; adulthood (rare)
In 5% associated with: ASD, VSD, aortic coarctation
Location: LCA arises from left inferolateral aspect of main pulmonary artery just beyond pulmonary valve
Hemodynamics:
postnatal fall in pulmonary arterial pressure → perfusion of LCA drops (= “coronary steal”) → L-to-R shunt (= collateral circulation from RCA with flow reversal in LCA)
› adequate collateral circulation = lifesaving
› inadequate collateral circulation = myocardial infarction
› large collateral circulation = volume overload of heart
• ECG: anterolateral infarction
√ LCA arising from the main pulmonary artery (HALLMARK) along its left inferolateral aspect just beyond pulmonic valve
√ coronary steal into PA
√ left ventricular wall motion abnormalities, mostly global hypokinesis
| Rx: | (1) Ligation of LCA at its origin from pulmonary trunk |
(2) Ligation of LCA + graft of left subclavian artery to LCA | |
(3) Creation of an AP window + baffle from AP window to ostium of LCA |
DDx: endocardial fibroelastosis; dilated cardiomyopathy; viral cardiomyopathy (NO shocklike symptoms)
A. INFANT TYPE
• symptomatic about 8 weeks after birth:
• failure to thrive, profuse sweating, dyspnea, pallor
• atypical chest pain while eating / crying
√ massive cardiomegaly during newborn period:
√ dilatation of LV ← chronic myocardial ischemia
√ enlargement of LA
√ congestive heart failure
√ mitral insufficiency ← myocardial infarction
√ normal pulmonary vascularity / redistribution
Prognosis: if untreated death within 1st year (in up to 90%)
B. ADULT TYPE (rare)
= sufficient right-to-left coronary artery collaterals
• asymptomatic, continuous murmur (if collaterals large)
• ischemic cardiomyopathy, malignant dysrhythmia
√ massively enlarged and tortuous collateral circulation from RCA to LCA ← increased flow:
√ abundant intercoronary collaterals on epicardial surface
√ development of dilated bronchial artery collaterals
Cx: chronic left ventricular subendocardial ischemia → malignant ventricular dysrhythmia
Prognosis: sudden cardiac death (in 80–90%)
ANOMALOUS PULMONARY VENOUS RETURN
= complete / partial failure of developing lung to connect with primitive common pulmonary vein retaining connections to primitive splanchnic system of cardinal veins (L-to-R shunt)
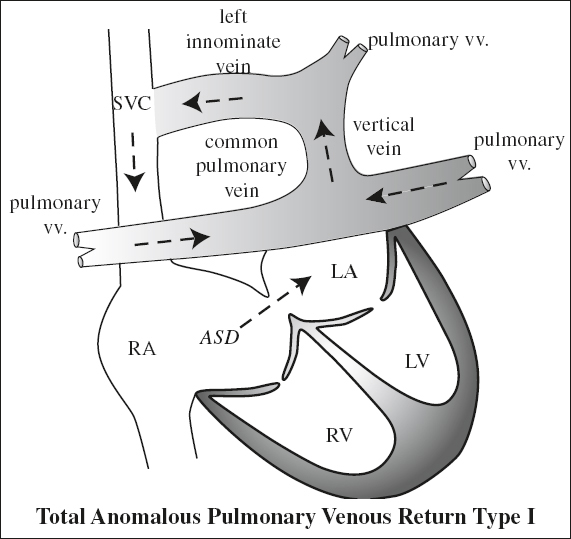
Total Anomalous Pulmonary Venous Return
= TAPVR = entire pulmonary venous return directed to RA = admixture lesion because of the combination of cyanosis + increased pulmonary vascularity (L-to-R and R-to-L shunt) = absent connection of pulmonary veins with LA
Embryology: persistence of primitive splanchnic pulmonary veins connecting to fetal cardinal systemic veins + failure of primitive common pulmonary vein to develop from posterior LA wall
◊ Usually isolated defect!
May be associated with:
asplenia; ASD / patent foramen ovale (necessary for survival); bronchopulmonary sequestration; pulmonary arteriovenous malformation; cystic adenomatoid malformation
Types: according to termination of anomalous pulmonary veins
| I | supracardiac level | 55% |
| II | cardiac level | 30% |
| III | infracardiac / infradiaphragmatic level | 13% |
| IV | termination at ≥ 2 levels (= mixed type) | 2% |
Prevalence: 2% of CHD
Age: symptomatic in 1st year of life
• cyanosis and congestive heart failure typically develop in the early neonatal period
Hemodynamics:
obstruction along the pulmonary venous pathway

MR:
√ “retroatrial” sign = presence of veins posterior to LA
√ small LA without pulmonary venous connections
√ patent foramen ovale / ASD
√ anomalous vein with variable location
In most potentially clinically unstable neonates with TAPVR, complete anatomic delineation of the defect is possible with echocardiography alone!
Overall prognosis: 75% mortality rate within 1 year of birth if untreated
Supradiaphragmatic TAPVR (85%)
Hemodynamics:
› functional L-to-R shunt from pulmonary veins to RA
› increased pulmonary blood flow (= overcirculation)
› obligatory R-to-L shunt via usually patent foramen ovale / ASD → restores oxygenated blood to left side
› normal systemic venous pressure with increased flow through widened SVC
› after birth CHF secondary to
(a) mixture of systemic + pulmonary venous blood in RA
(b) volume overload of RV
Age: presentation < 1 years of age
In 30% associated with: other cardiac lesions, asplenia
• cyanosis, neck veins undistended (shunt level distally)
• R ventricular heave (= increased contact of enlarged RV with sternum)
• systolic ejection murmur (large shunt volume)
√ overall heart size notably normal:
√ slightly enlarged RV (= volume overload with time)
√ normal / enlarged RA
√ normal LA (= ASD acts as escape valve)
√ increased pulmonary blood flow (= overcirculation)
SUPRACARDIAC TAPVR (55%)
= drainage of pulmonary veins into a horizontally oriented confluence posterior to LA → ascending vertical vein posterior to left atrial appendage + usually anterior to left pulmonary a. → left brachiocephalic (innominate) vein / right or left persistent SVC / azygos vein
√ “figure of 8” / “snowman” configuration of cardiac silhouette:
√ “head of snowman” = dilated vertical vein on left + innominate vein on top + SVC on right
√ “body of snowman” = heart with enlarged RA
√ pretracheal density on lateral film (= left vertical vein)
= 4 anomalous pulmonary veins converge behind LA forming a common vertical vein, which passes anterior to left PA + left main bronchus to join
(a) innominate vein (most commonly)
(b) left brachiocephalic vein
(c) right SVC
(d) azygos vein
√ extrinsic venous obstruction if vertical vein courses between left PA anteriorly + left main bronchus posteriorly (10%)
CARDIAC TAPVR (30%)
= drainage of pulmonary veins into coronary sinus (80%) / RA / SVC / (often obstructed) azygos vein
ECHO:
√ “whale’s tail” appearance ← dilated pulmonary veins and coronary sinus
Sub- / Infradiaphragmatic / Infracardiac TAPVR (13%)
= drainage of pulmonary veins into vertically oriented confluence posterior to LA → portal vein / ductus venosus / IVC / hepatic veins / left gastric vein; R-to-L shunt through ASD
Age: presentation in neonatal period with severe CHF
• intense cyanosis + respiratory distress
Hemodynamics: constriction of descending pulmonary vein by diaphragm en route through esophageal hiatus → (in > 90%) pulmonary venous hypertension + RV pressure overload
Associated with: asplenia syndrome (80%), polysplenia
√ pulmonary veins join to form a common vertical descending vein (“inverted fir tree”) posterior to LA:
√ vertical vein lies anterior to esophagus + descends through esophageal hiatus
√ low anterior indentation on barium-filled esophagus
√ connects (most commonly) to portal vein at confluence of splenic v. + superior mesenteric v.
√ unique appearance of pulmonary edema + pulmonary venous congestion with normal-sized heart (DDx: hyaline membrane disease)
√ thymic atrophy + depression of diaphragm
Cx: obstruction of pulmonary venous return in > 90%
Prognosis: death within a few days of life
Mixed Type of TAPVR (2%)
= with various connections to R side of heart (6%) at ≥ 2 levels, most commonly (a) vertical vein drains into left innominate (brachiocephalic) vein (b) anomalous vein(s) from right lung drain into RA / coronary sinus
Partial Anomalous Pulmonary Venous Return
= PAPVR = ≥ 1 pulmonary veins drain into systemic venous system / RA rather than LA (= L-to-R shunt)
Embryology: early atresia / malposition of central pulmonary v.
Prevalence: 0.3–0.7% of patients with CHD
Age: presentation later in life than TAPVR
N.B.: venous return almost never obstructed!
May be associated with: may occur in isolation
(1) Atrial septal defect (25%)
(a) RUL pulmonary vein (66%) enters SVC, RA, azygos vein, coronary sinus, IVC
Age: more common in children
◊ in 90% of patients with sinus venosus type ASD
◊ 50% of patients with PAPVR have a high sinus venosus type ASD near orifice of SVC
√ RUL vein courses in a horizontal direction
(b) LUL pulmonary vein (33%) enters brachiocephalic vein / coronary sinus
Age: more common in adults as incidental finding
Frequently associated with: ostium secundum type ASD (10–15%)
√ vertical mediastinal density lateral to aortic knob extending upward and medially with smooth curvilinear border (DDx: persistent left SVC)
(2) Hypogenetic lung as a component of congenital pulmonary venolobar / scimitar syndrome
• acyanotic and often asymptomatic
• ASD symptomatology (if ≥ 50% of pulmonary venous flow)
√ radiographic findings similar to ASD
√ anomalous course of draining vein
√ enlargement of draining site: SVC, IVC, azygos vein
CECT:
√ nodular / tubular opacity (= anomalous vein), which opacifies in phase with pulmonary vein
Prognosis: near normal life expectancy
Scimitar Syndrome
= HYPOGENETIC LUNG SYNDROME = CONGENITAL PULMONARY VENOLOBAR SYNDROME
[ scimitar = Persian or Turkish sword with curved blade]
= lower part / all of the hypogenetic lung is drained by an anomalous vein → L-to-R shunt
Hemodynamics: insignificant L-to-R shunt
Components: systemic supply + drainage of RLL
(1) Anomalous arterial supply of right lower lobe from abdominal aorta = systemic arterialization of the lung without sequestration (= pseudosequestration)
(2) Scimitar vein
(3) Hyopoplasia of right lung with dextroposition of heart:
(4) → Hypo- / aplasia of right pulmonary artery
Associated with: CHD (47–90%, most commonly ASD), extralobar sequestration, horseshoe lung, pulmonary AVM, bronchogenic cyst, accessory diaphragm
◊ High incidence of sinus venosus ASD with right upper lobe PAPVR
• asymptomatic (for shunt of < 50% of pulmonary flow) / pulmonary to systemic blood flow (Qp÷Qs) ≥ 1.5÷1
• acyanotic (until pulmonary arterial HTN occurs)
• heart murmur, fatigue, dyspnea
Location: almost exclusively on right side (1 case on left)
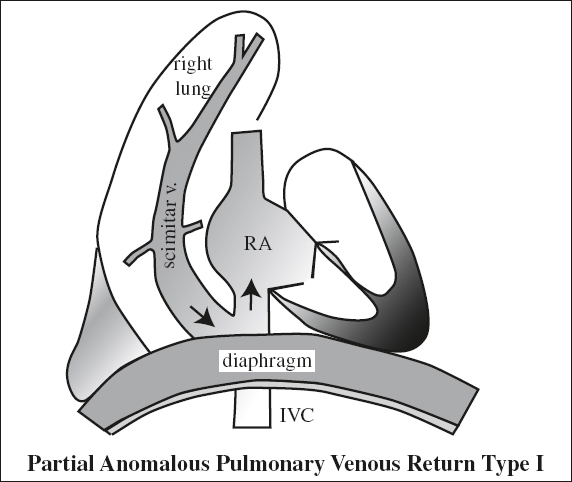
√ tubular structure paralleling the right heart border in an outward curved configuration of a Turkish sword increasing in diameter as the vein descends (PA view)
Site: middle of right lung to cardiophrenic angle
√ hypoplastic ipsilateral lung
√ small ipsilateral pulmonary hilum ← hypoplasia / aplasia of pulmonary artery
√ shift of heart + mediastinum into right chest
√ hyperinflated and hyperlucent contralateral lung
MR/CT:
The amount of L-to-R shunting is quantifiable by MR with cine phase-contrast flow analysis of the aorta and main pulmonary artery to estimate the Qp/Qs ratio!
√ systemic arterialization of right lung (without sequestration) ← anomalous artery from abdominal aorta / visceral artery
√ scimitar vein drains any / all lobes of right lung and connects to:
› infradiaphragmatic IVC (33%)
› suprahepatic IVC (22%)
› hepatic veins
› portal vein (11%)
› azygos vein
› coronary sinus
› right atrium (22%)
› left atrium = “meandering pulmonary vein”
◊ Drainage into suprahepatic portion of IVC / right atrium may be a clue for interruption of intrahepatic portion of IVC!
Prognosis: normal / near-normal life span
Rx: anomalous vein / veins anastomosed to LA + repair of ASD
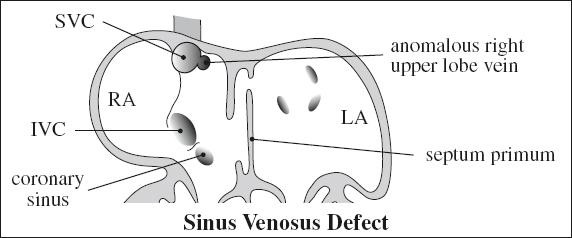
Sinus Venosus Defect
= SINUS VENOSUS–TYPE ATRIAL SEPTAL DEFECT
= unroofing of right pulmonary veins into SVC / IVC
N.B.: sinus venosus atrial septal defect is a misnomer since the true atrial septum is not involved
Types:
1. Sinus venosus defect of the SVC type
= unroofing of the right upper pulmonary vein (RUPV) into SVC = defect of the superior inlet portion of extraseptal atrial wall separating SVC–right atrial junction from posterosuperior aspect of LA = drainage of RUPV into SVC
Location: superior to fossa ovalis near entrance of superior vena cava (SVC straddles ASD)
2. Sinus venosus defect of the IVC type
= unroofing of the right lower pulmonary vein (RLPV) into IVC
variable: involvement of the right middle pulmonary vein (RMPV) can occur with either type
Function: R-to-L shunt similar to large ASD
• symptoms of right ventricular volume overload:
• fatigue, dyspnea, arrhythmia, heart murmur
Associated with:
partial anomalous pulmonary venous return in 90% (RUL pulmonary veins connect to SVC / right atrium), Holt-Oram syndrome, Ellis-van Creveld syndrome
AORTIC ANEURYSM
An aneurysm is defined as a vessel segment with a diameter of > 150% of the diameter of a normal adjacent segment + involving < 50% of the total vessel length!
Cause:
1. Aortic dissection (53%)
2. Atherosclerosis (29–80%): descending aorta; usually multiple
3. Traumatic (15–20%): descending aorta; ← transection
4. Congenital (2%): aortic sinus, post coarctation, ductus diverticulum
5. Syphilis (4%): ascending aorta + arch
6. Mycotic infection = bacterial dissection; anywhere
7. Cystic media necrosis (Marfan / Ehlers-Danlos syndrome, annuloaortic ectasia): ascending aorta
8. Aortitis = inflammation of media + adventitia: Takayasu arteritis, giant cell arteritis, relapsing polychondritis, rheumatic fever, rheumatoid arthritis, ankylosing spondylitis, Reiter syndrome, psoriasis, ulcerative colitis, systemic lupus erythematosus, scleroderma, Behçet disease, ulcerative colitis, radiation
9. Increased pressure: systemic hypertension, aortic valve stenosis
10. Abnormal volume load: severe aortic regurgitation
Pathophysiology:
intimal injury of aortic wall → lipid-laden macrophage + lymphocyte infiltration → inflammatory cells secrete cytokines → activation of multiple proteolytic cascades → degradation of collagen + elastin within tunica media → weakening + dilatation of aortic wall
Location: abdominal aorta (31%), ascending aorta (22%), arch (12%), descending thoracic aorta (8%), thoracoabdominal (3%)
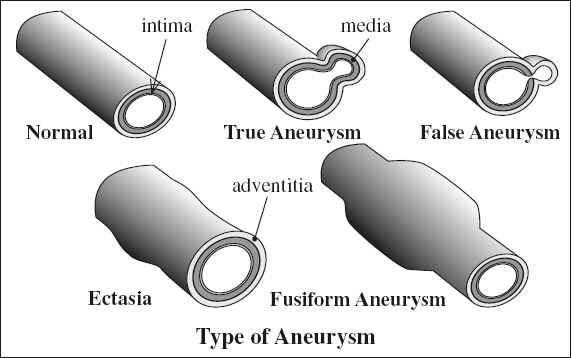
TRUE ANEURYSM
= permanent dilatation of all 3 layers (intima + media + adventitia) of weakened but intact wall
Fusiform Aneurysm (80%)
= longitudinal measurement > transverse diameter
Cause: atherosclerotic degeneration
√ circumferential involvement of wall over longer arterial segment
√ NO definable neck
√ NO relation to proximal stenosis
Saccular Aneurysm (20%)
= transverse diameter > longitudinal measurement
Cause: penetrating ulcer / inflammation / infection
√ berry-like outpouching of portion of wall
√ frequently distal to area of proximal stenosis
√ often multisegmental
Cx: prone to thrombosis and rupture
FALSE ANEURYSM = PSEUDOANEURYSM
= loss of wall integrity with transition from 3-layered wall to an outwardly single/double layer
Cause: trauma, penetrating atheroclerotic ulcer, infection (= “mycotic” aneurysm)
Pathophysiology: escaped blood contained by adventitia / perivascular connective tissue + organized blood
Histo: disruption of the external elastic membrane
√ saccular dilatation with a narrow neck
ARTERIAL ECTASIA
= diffuse dilatation
| Type I | diffuse dilatation of 2–3 vessels |
| Type II | ectasia of 1 vessel + aneurysm in another |
| Type III | solitary ectatic vessel |
Abdominal Aortic Aneurysm (AAA)
= focal widening > 3 cm (ultrasound literature); twice the size of normal aorta / > 4 cm [Bergan, Ann Surg 1984]
Normal size of abdominal aorta > 50 years of age:
14–21 [12–19] mm in men [women]
Prevalence: 1.4–8.2% in unselected population; in 6% > 80 years of age; in 6–20% of patients with signs of atherosclerotic disease; M > F; Whites÷Blacks = 3÷1
Cause: structural defect; ? genetic (10-fold ↑ in risk as 1st– degree relative of patient with AAA); copper deficiency
Risk factors: male sex, age > 75 years, white race, prior vascular disease, hypertension, cigarette smoking, family history, hypercholesterolemia
Age: > 60 years; M÷F = 5–9÷1
Associated with:
(a) visceral + renal artery aneurysm (2%)
(b) isolated iliac + femoral artery aneurysm (16%):
common iliac (89%), internal iliac (10%), external iliac (1%)
(c) stenosis / occlusion of celiac trunk / SMA (22%)
(d) stenosis of renal artery (22–30%)
(e) occlusion of inferior mesenteric artery (80%)
(f) occlusion of lumbar arteries (78%)
Growth rate of aneurysm of 3–6 cm in diameter:
0.39 cm annually
• asymptomatic (30%), abdominal mass (26%) / pain (37%)
◊ Imaging should provide information about:
(a) anatomic considerations of aneurysm:
» size
» proximal extent, which determines the site of clamping of the aorta (eg, origin of renal arteries)
» course of the left renal vein (retroaortic?)
(b) aneurysm growth rate
(c) findings of aneurysm instability
Location: infrarenal (91–95%) with extension into iliac arteries (66–70%)
◊ The infrarenal abdominal aorta has a lower concentration of elastin + vasa vasorum making it vulnerable to aneurysm formation!
Plain film:
√ mural calcification (75–86%)
US:
√ > 98% accuracy in size measurement
NCCT:
√ perianeurysmal fibrosis (10%) → ± ureteral obstruction
CECT:
(a) ruptured aneurysm
√ anterior displacement of kidney
√ extravasation of contrast material
√ fluid collection / hematoma within posterior pararenal + perirenal spaces
√ free intraperitoneal fluid
√ perirenal “cobwebs”
(b) contained leak
√ laminated mural calcification
√ periaortic mass of mixed / soft-tissue density
√ lateral “draping” of aneurysm around vertebral body
√ indistinct aortic wall (unreliable)
√ focal discontinuity of calcifications (unreliable)
A patchy discontinuous intimal calcification is common in stable and unstable aneurysms.
Angio (AP + LAT filming):
√ focally widened aortic lumen > 3 cm
√ apparent normal size of lumen ← mural thrombus (11%)
√ mural clot (80%)
√ slow antegrade flow of contrast medium
Cx:
(1) Aortic rupture (25%)
(2) Peripheral embolization
(3) Infection
(4) Spontaneous occlusion of aorta
Prognosis: 17% 5-year survival without surgery, 50–60% 5-year survival with surgery
| Rx: | (1) Excision of aneurysm + aortic interposition graft (4–5% surgical mortality for nonruptured, 30–80% for ruptured aneurysm) |
(2) Endovascular aneurysm repair (70%) with post-repair complications of graft migration > 5 mm, graft kinking / fracture, persistent endoleak |
Postoperative Cx:
(1) Left colonic ischemia (1.6%) with 10% mortality
(2) Renal failure (14%)
(3) 0–8% mortality rate for elective surgery
Acute Aortic Aneurysm Rupture
◊ Leading cause of death (4,500 annually) in USA in 1.3% of men > 65 years
Risk factors: female sex (M÷F = 1÷4), larger baseline aneurysm diameter, hypertension, continued tobacco use, low 1-second forced expiratory volume, history of cardiac / renal transplant
Risk for aneurysm rupture:
directly related to aneurysm size + rate of enlargement
Increased risk of aneurysm rupture: size > 6 cm
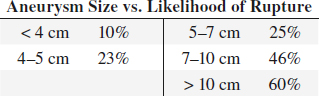
› growth > 5 mm / 6 months
The most accurate + reproducible bidimensional aneurysm measurements are those that are orthogonal to a center line through the aorta.
• pain + tenderness
◊ The exact moment of rupture is unpredictable
• sudden severe abdominal pain ± radiating into back
• faintness, syncope, hypotension
Site:
(1) into retroperitoneum: commonly on left
(2) into GI tract: massive GI hemorrhage
(3) into IVC: rapid cardiac decompensation
CT:
√ high-attenuation crescent within mural thrombus = acute contained rupture / impending rupture
√ retroperitoneal hematoma ± extension into perirenal space / pararenal space / psoas muscle / peritoneum
CECT:
√ active extravasation into thrombosed portion
Prognosis: 64–94% die before reaching hospital
Rx: most vascular surgeons electively repair a typical fusiform abdominal aortic aneurysm if > 5.4 cm
Chronic Contained Aortic Aneurysm Rupture
= aortic wall no longer fully intact with hemorrhage enclosed by a thrombus and/or retroperitoneal soft tissues
• hemodynamically stable
• ± previous episode of abdominal pain
Angio:
√ absent parenchymal stain = avascular halo
√ displacement + stretching of aortic branches
CT:
√ loss of periaortic fat planes:
√ commonly posterolateral focal area of soft-tissue attenuation intimately associated with aorta
√ “draped aorta” sign = posterior aortic wall not identifiable as distinct from adjacent structures / closely apposed to adjacent vertebral body contour
√ new saccular outpouching (= focal breach) of aneurysm wall in the region that appears draped on AXIAL view
√ anterior vertebral body scalloping ← chronic repetitive aortic pulsations and pressure
N.B.: contained rupture is unrelated to aneurysm size
Impending Aortic Aneurysm Rupture
A focal discontinuity of a circumferential calcified intimal plaque and outward displacement may indicate a contained rupture at NECT. A contained rupture is definite if the finding is new.
√ increasing size of aneurysm
Laplace law:
T (circumferential wall tension) = P (transmural pressure) • r (vessel radius)
√ thinning of thrombus = decreasing thrombus volume with progressive enlargement of flow lumen ← lysis of thrombus (thrombus protects against rupture)
√ focal discontinuity in circumferential wall calcifications ← focal plaque erosion = unstable aneurysm
√ “crescent” sign = periluminal curvilinear area of hyperattenuation in aneurysm wall / thrombus (= acute intramural hematoma) is 93% specific:
√ attenuation higher than intraluminal blood on NECT
√ attenuation higher than psoas muscle on CECT
√ ± perianeurysmal fat stranding
With bolus tracking a large aneurysm may not fully opacify, which can result in suboptimal enhancement of the aortic branches.
Extravasation indicative of active hemorrhage may not be apparent in a large rupturing aneurysm during the early arterial phase but rather during the late arterial / venous phase after the aneurysm has filled with contrast.
Indication for surgical repair:
√ diameter of > 5–6–7 cm
√ enlargement rate of ≥ 10 mm annually
Atherosclerotic Aneurysm of Abdominal Aorta
◊ There is no consensus regarding the definition of an atherosclerotic AAA!
Frequency: most common cause of aortic aneurysms; leading cause of thoracic aortic aneurysm
Histo: diseased intima with secondary degeneration + fibrous replacement of media → ultimately wall of aneurysm composed of acellular + avascular connective tissue
Pathophysiology:
progressive weakening of media → vessel dilatation + increased tension of vessel wall (law of Laplace = tensile stress varies with product of blood pressure and radius of vessel); compromise of mural vascular nutrition (vasa vasorum) → further degeneration + progressive dilatation
Age: elderly; M > F
• asymptomatic (most)
• chest pain; symptoms related to compression of adjacent structures (dysphagia, hoarseness, lobar atelectasis, pneumonia, parenchymal hemorrhage, superior vena cava syndrome)
Location: distal abdominal aorta (66%) > iliac a. > popliteal a. > common femoral a. > aortic + descending thoracic aorta > carotid a. > ascending aorta
Site:
(1) Infrarenal aorta (associated with thoracic aneurysm in 29%)
(2) Descending thoracic aorta distal to left subclavian artery
(3) Thoracoabdominal aorta
√ fusiform (80%), saccular (20%)
√ frequently contain calcified thrombus with irregular inner contour
Cx: rupture (cause of death in 50%): usually unrestrained + fatal in thoracic location
Degenerative Aneurysm
= medial degeneration
Most common cause of aneurysm in ascending aorta
Cause:
(1) Genetically transmitted metabolic disorder: Marfan syndrome, Ehlers-Danlos syndrome
(2) Acquired: result of repetitive aortic injury + repair associated with aging
Idiopathic Inflammatory Aortic Aneurysm
= defined as triad of
(1) thickened wall of aneurysm
(2) extensive perianeurysmal + retroperitoneal fibrosis
(3) dense adhesions of adjacent abdominal organs
Cause: slow leakage from aneurysm related to periaortic retroperitoneal fibrosis and autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, giant cell arteritis)
Frequency: 5–25% of all AAAs; rare in ascending aorta + aortic arch
Mean age: 62–68 years; M÷F = 6÷1 to 30÷1
• abdominal / back pain; weight loss + anorexia (20–41%)
• elevated ESR (40–88%), fever
• tender pulsatile abdominal mass (15–30%)
Comorbidities: arterial hypertension (34–69%), arterial occlusive disease (10–47%), diabetes mellitus (3–13%), coronary artery disease (33–55%)
Size: usually small at presentation because of early symptomatology
CECT (83% sensitive, 99% specific):
√ rind of homogeneous soft-tissue density surrounding aorta anteriorly + laterally sparing posterior wall
√ delayed contrast enhancement of soft-tissue component (DDx from hematoma)
MR:
√ periaortic inflammation and adventitial fibrosis
√ turbulent intraluminal flow
US:
√ sonolucent halo around aorta
PET:
√ grading extent of inflammation
| Cx: | (1) Entrapment of ureters (10–21%) + hydronephrosis |
(2) Aortic-sigmoid colon fistula + bleeding | |
(3) Secondary bacterial infection (eg, Salmonella) | |
(4) Enlargement + rupture irrespective of size (lower rate than in noninflammatory aneurysm) |
Prognosis: 23% mortality during surgical repair
Leaking Aortic Aneurysm
• acute chest pain
At risk for rupture: symptomatic > asymptomatic aneurysm; mycotic aneurysm; thoracic aortic aneurysm > 6 cm
MR:
√ irregular aneurysm wall
√ extra-aortic blood
√ pleural effusion containing high SI on T1WI (methemoglobin)
√ admixture of lower-intensity blood products + fat in mediastinum
Cx: rupture into left pleural space (descending thoracic aorta); rupture into pericardium/mediastinum (ascending thoracic aorta)
Mycotic (Infected) Aneurysm
[mycotic = misnomer used by Osler in 1885 describing a mushroom-shaped aneurysm associated with endocarditis]
= false (majority) / true aneurysm that is prone to rupture
Frequency: 0.7–2.6% of all aortic aneurysms
Age: > 5th decade; M > F
| Location: | (a) aorta: suprarenal (70%) > infrarenal > descending thoracic > thoracoabdominal > juxtarenal > ascending aorta near sinus of Valsalva |
(b) other arteries: abdominal visceral artery > intracranial artery > lower / upper extremity a. |
A mycotic aneurysm of the aortic root and sinus of Valsalva may be associated with infectious endocarditis, uni- / bicuspid aortic valve, and infected prosthetic aortic valve!
A. PRIMARY MYCOTIC ANEURYSM (rare) unassociated with any demonstrable intravascular inflammatory process
B. SECONDARY MYCOTIC ANEURYSM
= aneurysm due to nonsyphilitic infection
Predisposing factors:
(1) Atherosclerosis
(2) Aortic trauma ← accidents, aortic valve surgery, arterial graft, coronary artery bypass surgery, intravascular catheter, arterial catheterization, joint prosthesis
Risk factors:
(1) IV drug abuse
(2) Sepsis
(3) Bacterial endocarditis (12%)
(4) Immunocompromise (malignancy, alcoholism, corticosteroid therapy, chemotherapy, autoimmune disease, diabetes)
Mechanism:
(a) hematogenous:
› septicemia with abscess formation via vasa vasorum
› septicemia with abscess formation via vessel lumen
(b) direct extension from adjacent infection: osteomyelitis of sternum or spine, renal or psoas abscess → weakening + destruction part of the aortic wall
(c) traumatic / iatrogenic: intima laceration (trauma, atherosclerosis, coarctation)
Organism: S. aureus (53%) > E. coli > Salmonella (33-50%) > nonhemolytic Streptococcus > Listeria > Haemophilus, Pneumococcus, Gonococcus, Mycobacterium (contiguous spread from spine / lymph nodes)
Histo: loss of intima + destruction of internal elastic lamella; varying degrees of destruction of muscularis of media + adventitia
• frequently insidious → late-stage septic shock
• persistent fever, leukocytosis ← bacteremia (positive blood culture in only 53%); NO acute chest pain
Size: 1–11 cm
√ CHARACTERISTIC saccular formation (> 90%) with lobular contour arising eccentrically from aortic wall
√ rapid enlargement (faster expansion rate compared with atherosclerotic aneurysm as short as 7 days)
√ interrupted ring of aortic wall calcification
√ periaortic fat stranding / fluid / gas collection
√ adjacent vertebral / sternal osteomyelitis (rare)
√ periaortic / psoas abscess
√ adjacent reactive lymph node enlargement
| Cx: | (1) Life-threatening rupture + hemorrhage (50–75%) |
(2) Uncontrolled sepsis if untreated |
Rx: prompt surgery
Prognosis: 67% overall mortality
Syphilitic Aneurysm
= sexually transmitted chronic systemic infection caused by spirochete Treponema pallidum
= tertiary stage of syphilis consisting of neurosyphilis, gumma, cardiovascular involvement
Spectrum:
1. Uncomplicated syphilitic aortitis
2. Syphilitic aortic aneurysm (mostly saccular)
3. Syphilitic aortic valvulitis (aortic regurgitation)
4. Syphilitic coronary ostial stenosis
N.B.: dissection (uncommon) ← scarring of media
Frequency: 12% of patients with untreated syphilis
Onset: 5–30 years after initial spirochete infection
Histo: chronic inflammation of vasa vasorum → obstruction of vasa vasorum → nutritional impairment of aortic media → focal destruction of aortic media with loss of elastic + smooth muscle fibers replaced by scar
• positive venereal disease research laboratory (VDRL) test
• positive microhemagglutination assay – Treponema pallidum (MHA-TP) test
Location: ascending aorta (36–60%), aortic arch (34%), proximal descending aorta (25%), distal descending aorta (5%), aortic sinuses (< 1%)
√ asymmetric enlargement of aortic sinuses (DDx to annuloaortic ectasia with symmetric enlargement)
√ saccular (75%) / fusiform (25%) aneurysm
√ TYPICAL pencil-thin dystrophic aortic wall calcification (up to 40%) most severe in ascending aorta, frequently obscured by thick coarse irregular calcifications of secondary atherosclerosis
√ early sternal erosion affecting mainly right side of manubrium + medial end of right clavicle
√ narrowing of coronary ostia (subintimal scarring)
Prognosis: death ← aortic rupture in 40%; death ← myocardial infarction within 6–8 months of onset of symptoms if untreated
Rx: penicillin
Thoracic Aortic Aneurysm
Most common vascular cause of mediastinal mass!
◊ 10% of mediastinal masses are of vascular origin!
Definitions:
diameter of 4 – 5 cm = aortic ectasia
diameter of > 5 cm = aortic aneurysm
Frequency: 25% of all aneurysms
Cause: atherosclerosis (29%), aortic dissection (53%), aortitis (8%), cystic medial necrosis (6%), syphilis (4%)
◊ Bicuspid aortic valve = independent risk factor!
Associated with: hypertension, coronary artery disease, abdominal aneurysm (30%)
Mean age: 65 years; M÷F = 3÷1
• substernal / back / shoulder pain (26%)
• SVC syndrome ← venous compression)
• dysphagia ← esophageal compression)
• stridor, dyspnea ← tracheobronchial compression)
• hoarseness ← recurrent laryngeal nerve compression)
Location: arch > descending aorta
√ mediastinal mass with proximity to aorta
√ wide tortuous aorta:
√ annual growth rate of 0.07–0.42 cm
√ curvilinear peripheral calcifications (75%)
√ circumferential / crescentic mural thrombus
N.B.: Angio may show normal caliber ← mural thrombus!
Cx:
(1) Rupture into mediastinum, pericardium, either pleural cavity, airway, esophagus
Median size at rupture-dissection:
5.9 cm for ascending aorta,
7.2 cm for descending aorta
√ high-attenuation fluid
(2) Aortobronchopulmonary fistula
√ consolidation of lung adjacent to aneurysm
◊ Most aneurysms rupture when > 10 cm in size
Prognosis: 1-year survival 57%; 3-year survival 26%; 5-year survival 19% (60% die from ruptured aneurysm, 40% die from other causes)
Rx: operative repair should be considered
for ascending aorta at > 5.5 cm
for descending aorta at > 6.5 cm
for Marfan syndrome > 5.0 cm
at annual growth rate > 1.0 cm
Surgical mortality: 9% for elective + 22% for emergent surgery
Annuloaortic Ectasia
= dilated sinuses of Valsalva with effacement of sinotubular junction
Cause: Marfan syndrome, idiopathic (30%), homocystinuria, Ehlers-Danlos syndrome, osteogenesis imperfecta
√ pear-shaped aorta
Valsalva Sinus Aneurysm
= aneurysm originating from a coronary sinus above aortic annulus
Prevalence: in 0.09% of autopsies; in 0.15%–3.50% of heart surgeries
Types:
(a) congenital: localized weakness of elastic lamina at junction of aortic media and annulus fibrosus in Marfan and Ehlers-Danlos syndrome
(b) acquired: infectious disease (bacterial endocarditis, syphilis, tuberculosis); degenerative (atherosclerosis, cystic medial necrosis); deceleration injury; aortic valve replacement
Associated with: supracristal VSD (30%–60% of patients), aortic insufficiency (20%–30%), bicuspid aortic valve (10%), coronary anomalies
Age: 35.4 (range, 4 days–96) years; M÷F = 2÷1 to 4÷1; Eastern/Asian÷Western countries = 5÷1
• asymptomatic (14%)
• cardiac murmur (57%), dyspnea (56%), chest pain
• insidiously progressive heart failure ← volume overload
| Location: | (a) right coronary sinus (72%) |
(b) noncoronary sinus (22% | |
(c) coronary sinus (6%) |
ECHO: diagnostic in 90%
Imaging criteria for a Valsalva sinus aneurysm include
(1) origin above aortic annulus
(2) saccular shape
(3) normal dimensions of adjacent aortic root and ascending aorta!
Cx:
(a) rupture (66%):
1. Aortic regurgitation (in 30–50%)
2. Aortocardiac shunt with RV (56%), RA (30%), RVOT (9%), LV (2%), interventricular septum (2%), LA (1%), extracardiac space (rare)
(b) mass effect:
1. Aortic regurgitation
2. Impaired function of tricuspid / mitral valve
3. Occluded / partially obstructed RVOT
4. Dissection into interventricular septum
5. Compression / occlusion of a coronary artery → myocardial ischemia
Surgery is indicated for asymptomatic patients without another underlying cardiovascular condition or disease, when aortic root measures ≥ 5.5 cm
DDx: prolapsing aortic cusp (below annulus)
Traumatic Aortic Pseudoaneurysm
= CHRONIC AORTIC PSEUDOANEURYSM
◊ 2nd most common form of thoracic aortic aneurysm
◊ Most common type occurring in young patients
Frequency: 2.5% of patients who survive initial trauma of acute aortic transection
√ usually calcified
√ may contain thrombus
| Cx: | (1) Progressive enlargement |
(2) Rupture (even years after insult) |
Complications of Endovascular Stent-Graft Repair
1. Endoleak (2–45%)
= leakage into the aneurysm outside stent-graft
Type 1 = incomplete fixation of stent-graft to aortic wall at the proximal / distal attachment site
Type 2 = retrograde flow via parent artery (eg, lumbar / inferior mesenteric artery) in up to 24%
◊ High rate of spontaneous resolution if aneurysmal sac size remains stable
Type 3 = endograft defect with disruption of either metallic support / fabric
Type 4 = porous graft (uncommon)
Type 5 = endotension = aneurysm expansion > 5 mm without demonstrable endoleak ( ← nonvisualized endoleak, ultrafiltration of blood across graft membrane, thrombus as ineffective barrier)
2. Graft kinking
Cause: diminishing diameter of aneurysm after stent-graft implantation also decreases length of aneurysm
Associated with: distal migration of stent-graft
3. Graft infection
√ interval development of perigraft soft-tissue attenuation / air
Rx: antibiotics + total excision of infected graft
4. Graft thrombosis (3–19%)
= intraluminal circular / semicircular thrombus
Prognosis: spontaneous shrinkage, development of complete thrombosis
5. Graft occlusion
6. Shower embolism (4–17%)
Cause: mural thrombus dispersed by delivery system
Prognosis: perioperative death
7. Colon necrosis
Cause: occlusion of inferior mesenteric a. by stent-graft
8. Aortic dissection
Cause: retrograde injury by delivery system
AORTIC DISSECTION
= spontaneous longitudinal separation of aortic intima and adventitia by circulating blood having gained access to and splitting the media of the aortic wall
◊ Most common ACUTE EMERGENCY condition of the aorta!
Incidence: 3÷1,000 (more common than all ruptures of thoracic + abdominal aorta combined); 1÷205 autopsies; 2,000 cases annually in USA
Peak age: 60 years (range 13–87 years); M÷F = 3÷1
Predisposed: cystic medial necrosis / disease of aortic wall
◊ Starts in fusiform aneurysms in 28%
◊ Does not occur in aneurysms < 5 cm in diameter
1. Hypertension (60–90%)
2. Marfan syndrome (5–16%)
3. Ehlers-Danlos syndrome
4. Turner syndrome
5. Noonan syndrome
6. Polycystic kidney disease
7. Osteogenesis imperfecta
8. Valvular aortic stenosis
9. Coarctation
10. Bicuspid aortic valve
11. S/P prosthetic valve / other cardiovascular procedure
12. Blunt trauma (rare)
13. Inflammatory collagen vascular disease
14. Relapsing polychondritis
15. Aortitis (eg, Takayasu disease, SLE)
16. Behçet disease
17. Patient on corticosteroids
18. Cocaine abuse
19. Family history of dissection
20. Personal history of aortic aneurysm (2–12%)
21. Atherosclerosis of aorta (24–42%)
22. Pregnancy
◊ In women 50% of dissections occur during pregnancy!
N.B.: NOT syphilis
Path: destruction of media leads to formation of a false channel:
(1) Transverse tear in weakened intima (95–97%)
◊ The diagnosis relies primarily on visualization of an intimal flap + blood flow within a false lumen
(2) Primary hemorrhage into aortic wall WITHOUT intimal tear (3–5–13%) = atypical noncommunicating aortic dissection
Pathogenesis:
intimal tear results from combination of the following factors:
(a) media degeneration decreases cohesiveness of aortic wall
(b) persistent motion ← beating heart stresses aortic wall
(c) hypertension accentuates hydrodynamic forces
The classic intimal flap is seen in ~ 70% of aortic dissections.
Pathophysiology:
luminal pressures:
intimal tear allows blood to enter media (= false lumen) with pressure in false lumen ≥ pressure in true lumen → collapse of true lumen + expansion of false lumen → possible compression + obstruction of true lumen;
degree of dilatation of false lumen depends on
(a) blood pressure
(b) depth of medial dissection plane,
(c) percentage of involved wall circumference;
flow direction in false lumen: antegrade / retrograde
effect on branch vessel:
(a) static obstruction = intimal flap enters branch-vessel origin without reentry point
(b) dynamic obstruction = intimal flap prolapses into / covers branch vessel like a curtain
fate of false lumen:
may remain patent / thrombose / recommunicate with true lumen through fenestrations / rupture into potential spaces (pericardial, pleural, peritoneal)
Definition of acute versus chronic dissection:
(a) acute aortic dissection = symptoms present for < 14 days
(b) chronic aortic dissection = symptoms present for ≥ 14 days
• sharp tearing intractable anterior / posterior chest pain (75–95%) radiating to jaw, neck, low back (DDx: myocardial infarction)
• murmur ± bruit (65%) from aortic regurgitation
• malperfusion symptoms:
• asymmetric peripheral pulses + blood pressures (59%)
• absent femoral pulses (25%), reappearing after reentry
• pulse deficit: in up to 50% of type A dissection, in 16% of type B dissection; persistent oliguria, anuria
• neurologic deficits (5–25%): hemiplegia, paraparesis ← compromise of anterior spinal artery of Adamkiewicz
• hemodynamic shock (25%)
• congestive heart failure (rare) ← acute aortic insufficiency:
• diastolic murmur (40–50%)
• recurrent arrhythmias / right bundle branch block
• signs of pericardial tamponade: clouded sensorium, extreme restlessness, dyspnea, distended neck veins
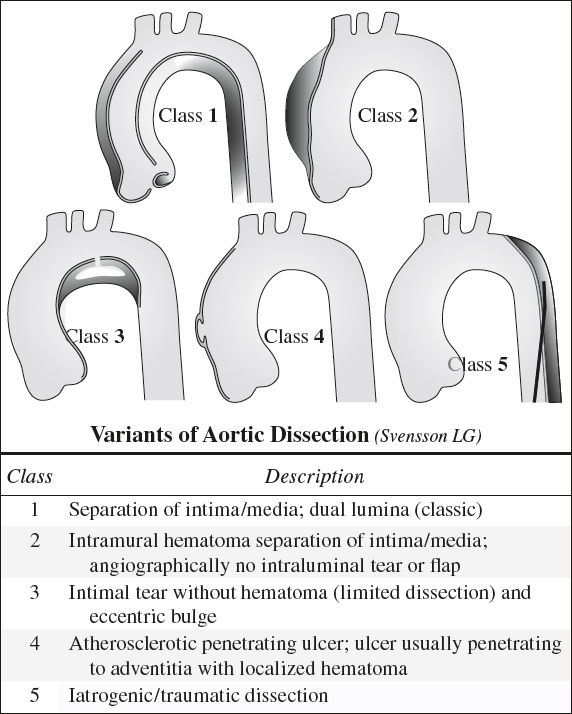
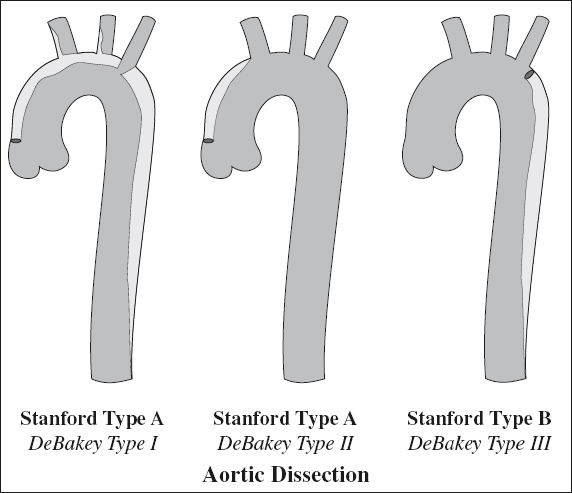
Types:
DeBakey Classification:
Type I (29–34%) = ascending aorta + portion distal to arch
Type II (12–21%) = ascending aorta only
Type III (50%) = descending aorta only
| subtype III A | = | up to diaphragm |
| subtype III B | = | below diaphragm |
Stanford Classification: based on need for surgical therapy
Type A (60–70%) = ascending aorta ± arch in first 4 cm
Type B (30–40%) = descending aorta only
mnemonic: A affects ascending aorta and arch; B begins beyond brachiocephalic vessels; I = II + III
Flow velocities (average):
• 13.4 cm/second in true lumen
• 3.1 cm/second in false lumen
• retrograde flow more common in false lumen
Location of dissection flap (following helical flow pattern):
› on right anterolateral wall of ascending aorta just distal to aortic valve (65%)
› on posterosuperior wall of transverse aortic arch (10%)
› on left posterolateral wall of upper descending aorta distal to left subclavian artery (20%)
› more distal aorta (5%) usually terminating in left iliac artery (80%) / right iliac artery (10%) [involvement of left renal artery in 50%]
◊ An exit / distal tear / reentry occurs in 10%!
Imaging characteristics of true lumen:
√ usually smaller and oval / semiround
√ in continuity with undissected portion of aorta
√ higher concentration of contrast
√ ribbonlike
Imaging characteristics of false lumen:
√ SPECIFIC “cobweb” sign = slender linear areas of low attenuation ← residual ribbons of media
√ “beak” sign at cross-sectional imaging = wedge of hematoma creating space for propagation of false lumen
√ rewinding around true lumen on axial plane
√ may become thrombosed
Atypical configurations of intimal flap:
√ circumferential intimal flap ← dissection of entire intima
√ filiform intimal flap creating an extremely narrow true lumen ± ischemic complications
√ mural calcification of false lumen (in chronic dissection)
√ three-channel aorta (= “Mercedes-Benz” sign) ← two false channels ← secondary dissection of one channel
◊ Multibarreled aortic dissections (9% of all aortic dissections) have a significantly poorer survival!
√ intimointimal intussusception → “windsock” appearance
Secondary signs of aortic dissection:
√ internal displacement of intimal calcification
√ delayed enhancement of false lumen
√ widening of aorta and mediastinum
√ pleural / pericardial hematoma
CXR (best assessment by comparison with serial films):
√ normal CXR in 10–25–40%
√ “calcification” sign = inward displacement of atherosclerotic plaque by > 4–10 mm from outer aortic contour (7%)
N.B.: can only be applied to contour of descending aorta due to projection; may be misleading in presence of periaortic soft-tissue mass / hematoma
√ disparity in size between ascending + descending aorta
√ irregular wavy contour / indistinct outline of aorta
√ widening of superior mediastinum to > 8 cm ← hemorrhage / enlarging false channel (40–80%)
√ cardiac enlargement ← LV hypertrophy / hemopericardium
√ left pleural effusion (27%)
√ atelectasis of lower lobe
√ rightward displacement of trachea / endotracheal tube
ECHO:
(a) transesophageal echocardiography (TEE): 95–100% sensitive + 77–97% specific
◊ Ultrasound can be performed at bedside!
False-positive (33%): reverberation artifacts from calcified aortic wall
(b) transthoracic US: 59–85% sensitive + 63–96% specific for type A dissection; poorer for type B
(c) intravascular US (in conjunction with aortography) to differentiate true from false lumen
√ intimal flap (seen in more than one view)
√ pericardial fluid
√ aortic insufficiency
False-positives: reverberation echoes from aneurysmal ascending aorta / calcified atheromatous plaque, postoperative periaortic hematoma
Angio (86–88% sensitive, 75–94% specific):
◊ Largely replaced by noninvasive cross-sectional imaging techniques
Superior to any other technique in demonstrating
› entry + reentry points (in 50%)
› branch vessel involvement + coronary arteries
› aortic insufficiency
√ visualization of intimal / medial flap (75–79%) = linear radiolucency within opacified aorta
√ “double barrel aorta” (87%) = opacification of two aortic lumens
√ abnormal catheter position outside anticipated aortic course
√ compression of true lumen by false channel (72–85%)
√ aortic valvular regurgitation (30%)
√ increase in aortic wall thickness > 6–10 mm
√ obstruction of aortic branches: left renal artery (25–30%)
√ ulcerlike projections caused by truncated branches
√ slower blood flow in false lumen
False-negative: complete thrombosis / very slow blood flow of false channel (10%), intimal flap not tangential to x-ray beam
False-positive: thickening of aortic wall due to aneurysm, aortitis, adjacent neoplasm / hemorrhage
NECT:
√ high attenuation within aortic wall 2° to:
(a) high-attenuation of false lumen (slow flow / thrombosis)
(b) presence of intramural hematoma
√ intimal flap separating two aortic channels (may be seen without contrast in anemic patients)
CECT (87–100% sensitive, 87–100% specific):
within 4 hours (if patient responds rapidly to medical Rx); detection as accurate as angio with single-level dynamic scanning (cardiac gating desirable to avoid overdiagnosis)
√ intimal flap separating two aortic channels
√ crescentic high-attenuation clot within false lumen
√ internally displaced intimal calcification (DDx: calcification of thrombus on luminal surface or within)
√ entry tear = most proximal split / discontinuity in intimal flap
False-negative: inadequate contrast opacification, thrombosed lumen misinterpreted as aortic aneurysm with mural thrombus
False-positive: perivenous streaks ← beam hardening + motion, cardiac / aortic motion artifacts; opacified normal sinus of Valsalva; normal pericardial recess mistaken for thrombus; mural thrombus in a fusiform aortic aneurysm; periaortic fibrosis; anemia with apparent high attenuation of aortic wall
MR (95–100% sensitive, 90–100% specific):
Advantage: large field of view in any plane; contrast material NOT necessary
Disadvantage: longer imaging time; difficulty monitoring acutely ill patients; image degradation from motion (uncooperative patient, atrial fibrillation)
SE images:
√ intimal flap of medium intensity outlined by signal voids of rapidly flowing blood in true + false lumen
√ intimal flap more difficult to detect in the presence of slow flow / thrombus ← false lumen has intermediate intensity instead of flow void
√ “cobwebs” (25%) traversing the corners of the false lumen = bands of medial elastic lamellae spanning the junction of the dissecting septum with outer wall of false lumen
GRE images:
√ lower-intensity intimal flap between high-intensity channels of flowing blood
√ intermediate signal from thrombosed lumen
√ aortic valve insufficiency = conical area of signal loss from aortic valve into LV during systole ← intravoxel dephasing caused by turbulence
Cx:
(1) Retrograde dissection (in Stanford type A)
(a) aortic insufficiency
(b) occlusion of coronary artery (8%)
(c) internal rupture into RV, LA, vena cava, pulmonary artery producing a large L-to-R shunt
(2) Occlusion / transient obstruction of major aortic branches (in up to 27%)
(a) static obstruction
√ flap enters branch-vessel origin
(b) dynamic obstruction = flap spares branch-vessel origin but covers it like a curtain
√ collapsed true lumen outlined by a C-shaped flap envelope which is concave toward false lumen (= ischemic configuration)
(3) External rupture of aorta into mediastinum, pleural cavity / pericardial sac (75%), right ventricle, left atrium, vena cava, pulmonary artery: 70% mortality (= most common cause of death within 24 hours)
NECT: √ hyperattenuating mediastinal / pericardial / pleural fluid collection
CECT: √ extravasation of vascular contrast material
(4) Development of aneurysm (15%) of the true / false lumen
◊ Organs may receive their blood supply through either the true or false lumen or both!
Rx:
(1) Reducing peak systolic pressure to 120–70 mmHg (adequate ONLY for type III = B, which rarely progresses proximally):
Cx: death from rupture of aortic aneurysm in 46% of hypertensive + 17% of normotensive patients
Survival rate: 40–70% with medical / surgical management
(2) Immediate surgical graft reinforcement of aortic wall (Type I, II = A) preventing rupture + extension into aortic root → progressive aortic valve insufficiency
Nonsurgical survival rate: < 10%
Postsurgical mortality: 10–35%
Cx: myocardial infarction, stroke, respiratory insufficiency, pulmonary embolism, aortic rupture, pseudoaneurysm, graft infection
Prognosis without Rx:
immediate death (3%); death within: 1 day (20–30%), 1 week (50–62%), 3 weeks (60%), 1 month (75%), 3 months (80%), 1 year (80–95%)
Prognosis with Rx:
5–10% mortality rate following timely surgery;
40% 10-year survival rate after leaving hospital
Death from thoracic aortic dissection may occur ← acute aortic regurgitation; ← major aortic branch obstruction; ← pericardial tamponade; ← aortic rupture into pericardium / left pleural cavity / mediastinum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree