Fig. 1
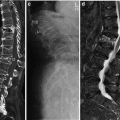
85-year-old woman with severe back pain from L1 fracture with mild retropulsion. Unilateral transpedicular kyphoplasty results in pain relief. CT of the lumbar spine, coronal (a) and sagittal (b) reconstruction images show fracture of the L1 vertebra with vacuum phenomenon and severe height loss. Axial imaging (c) also confirms retropulsion. X-ray (d) shows 70 % height loss (arrows). Kyphoplasty trocar (arrows) has been introduced into the vertebral body of L1 through a right transpedicular approach visible on AP (e) and lateral (f) views. AP (g) and lateral (h) views show that single balloon (arrow) positioned in the center of the vertebral body provides significant height recovery. AP (i) and lateral (j) views show that moderate reduction has been obtained with cement injection (arrows). The posterior wall is not compromised
Extreme compression fractures (vertebra plana) make the procedure technically difficult, although not impossible if the endplates are intact (Fig. 2). Traumatic fractures not associated with osteoporosis that cause recurrent severe pain may be considered for vertebroplasty, particularly if relatively recent and if surgery is not a reasonable consideration (Fig. 3). Kyphoplasty has also been reported in the successful treatment of a vertebral fracture associated with Guillain-Barré syndrome, promoting significant improvement in functional activity and neurological function by allowing the patient to enroll immediately in a rehabilitation program (Masala et al. 2004).
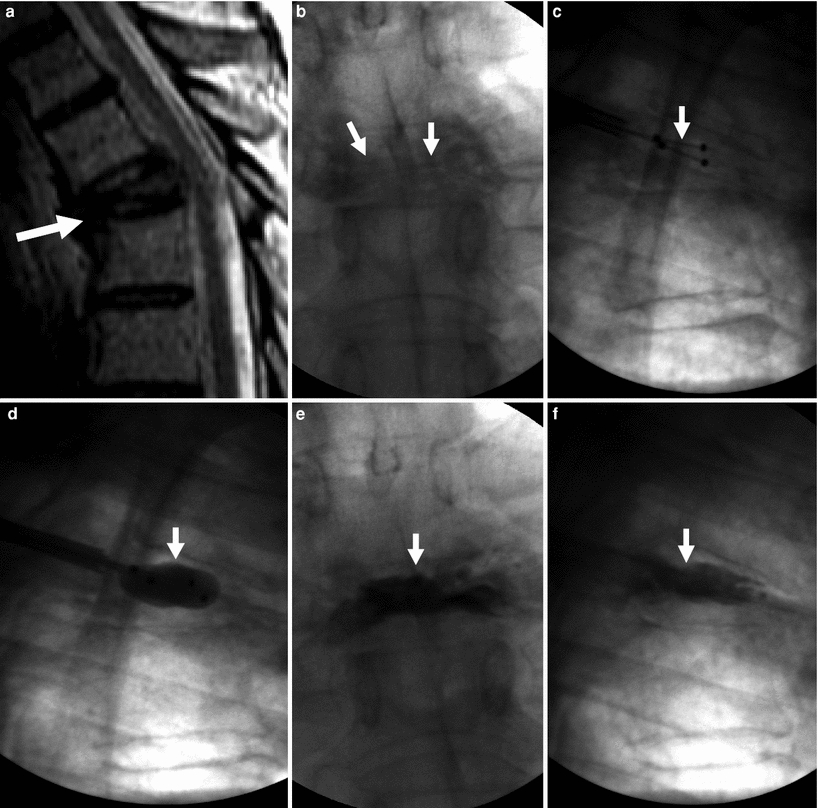
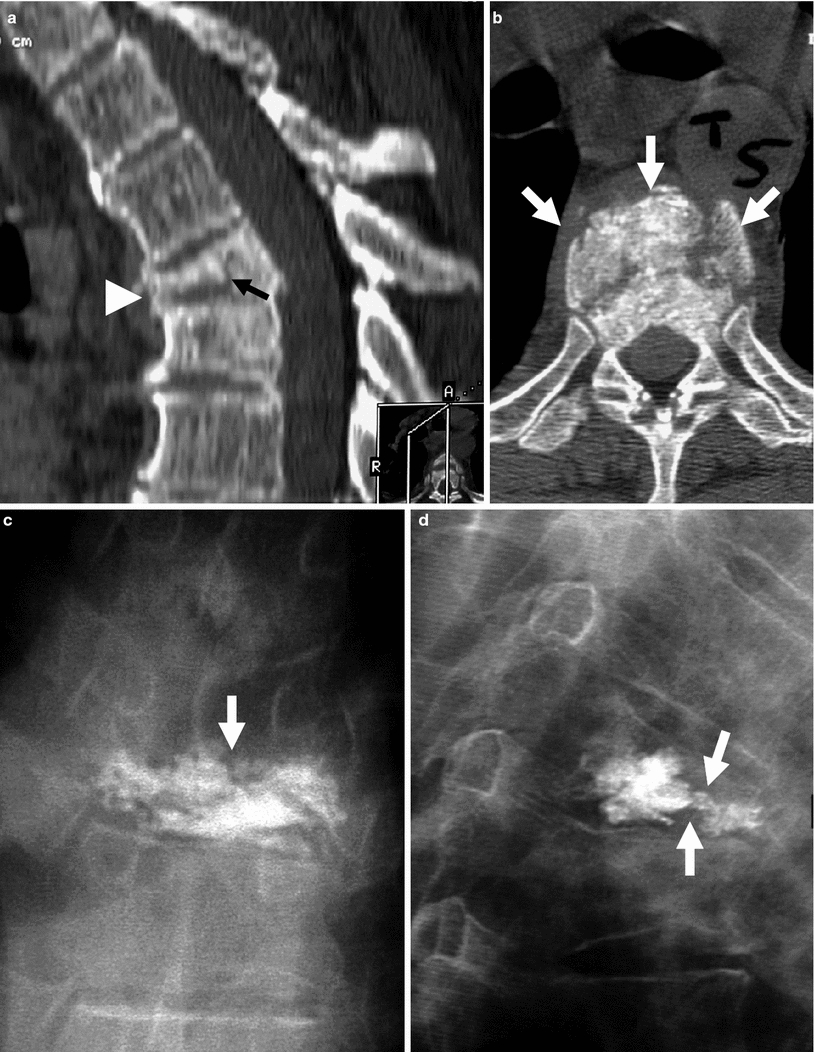

Fig. 2
Kyphoplasty for painful T5 vertebra plana fracture in a 67-year-old man with multiple myeloma. Excellent pain relief. a Sagittal T2 weighted MRI shows T5 fracture with severe anterior wedging and height loss (arrow) in a vertebra plana pattern. b X-ray, anteroposterior view shows severe height loss of T5 (arrows). c X-ray, lateral view shows deflated kyphoplasty balloons placed within the vertebral body of T5 (arrow). d X-ray, lateral view shows inflated kyphoplasty balloons within T5 (arrow). e X-ray, anteroposterior view shows excellent cement filling in T5 (arrow). f Lateral X-ray shows excellent reduction of T5 (arrow)

Fig. 3
Example of traumatic fracture in 66-year-old man treated with vertebroplasty with excellent pain relief. a Sagittal reconstruction of thoracic spine CT shows marked anterior wedging and height loss (arrowhead), and fracture line (arrow). b Axial imaging at T5 shows several fracture lines (arrows) in the vertebral body resulting in a “burst” pattern. c Anteroposterior view shows discontinuous cement deposition within the vertebral body (arrow). d Lateral view also shows irregular cement deposition in the vertebral body of T5, with apparent vertebral reconstruction and no posterior cement leakage (arrows)
3 Vertebroplasty, Kyphoplasty, and Newer Vertebral Augmentation Procedures
Vertebroplasty, the first vertebral augmentation procedure described, involved the direct injection of bone cement within the spongious bone of the vertebral body through needles inserted through one or both pedicles. The first variation of the technique was to use a unilateral transpedicular approach to increase the safety and decrease the duration of the procedure. Technical improvements to the vertebroplasty procedure soon took place, the most notable being curved and directional needles (Cook®, DePuy Osseon®, AvaFlex®), bone filler needles (CareFusion®, Stryker®), cavity creating devices (Latitude®), and newer cements with higher viscosity containing bioceramics (Cortoss®) or calcium phosphate hydroxyapatite (Actos®).
The concept of inserting and inflating a balloon into a vertebral body was first developed in the mid-1980s by an orthopedic surgeon, Dr. Mark Reiley and an engineer, Arie Scholten. It was not until 1994 that Dr. Reiley was able to find investors interested in his “balloons in bones” idea. The newly formed Kyphon Corporation (Sunnyvale, CA) succeeded in developing a balloon capable of displacing bone. The KyphX® Inflatable Bone Tamp™ received 510(k) clearance from the United States Food and Drug Administration (FDA) in July 1998 (Fig. 4).
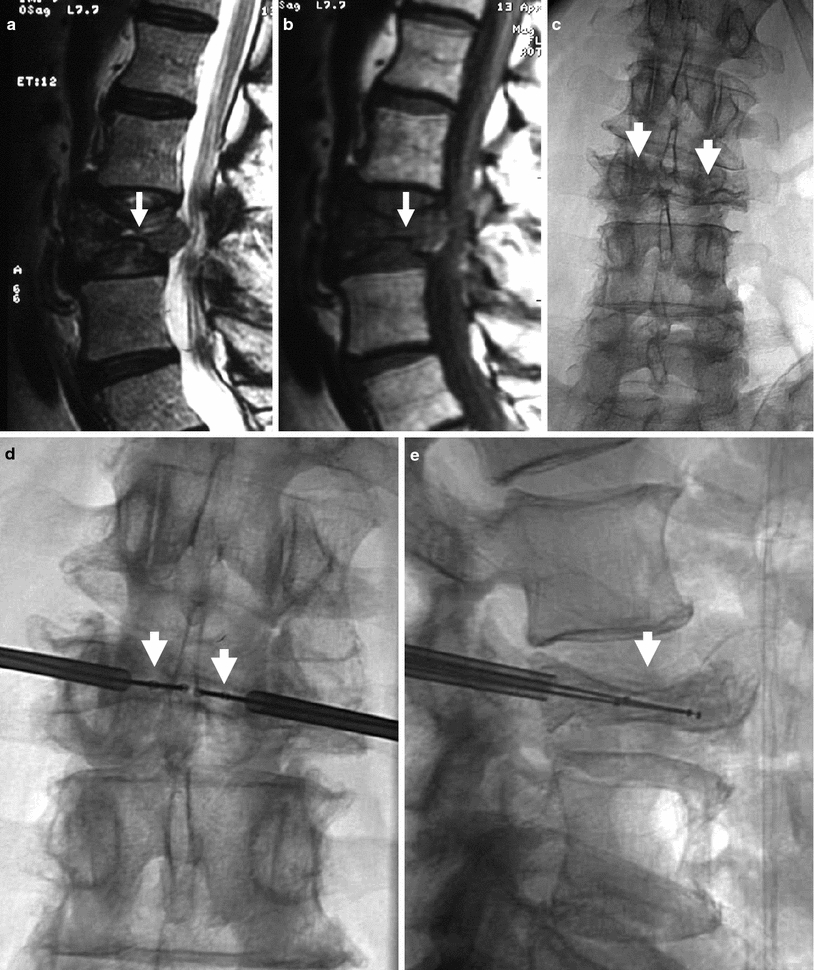
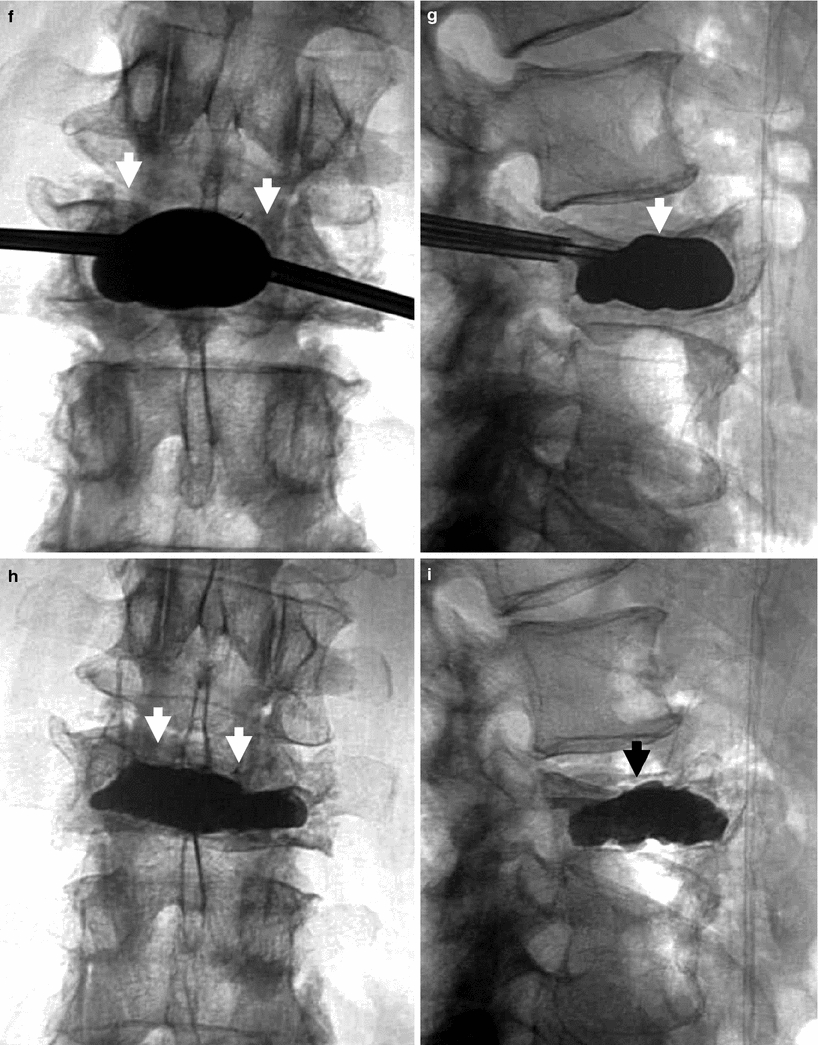


Fig. 4
Kyphoplasty using a bilateral transpedicular approach to treat an extremely painful fracture of L3 with significant retropulsion in a 75-year-old woman with scoliosis. a Sagittal T2 weighted MRI shows a hyperintense horizontal cleft in the superior aspect of the vertebral body of L3 (arrow). b Sagittal T1 weighted MRI shows hypointense signal within the cleft (arrow), confirming bone marrow edema. c X-ray of lumbar spine shows L3 fracture with 70 % height loss (arrows). d, e. Excellent placement of kyphoplasty balloons within L3 vertebral body (arrows). Note some degree of reduction of scoliosis. f, g Balloon inflation results in approximately 50 % height recovery (arrows). h, i After cement filling, there is approximately 30–40 % height recovery (arrows) with fracture reduction, and less pronounced scoliosis
Lordoplasty is a variation of vertebroplasty which reportedly has similar pain relief rates (in the 90 % range), and has the theoretical advantage of reducing vertebral and segmental kyphosis by 10–15° (Orler et al. 2006). Lordoplasty uses cannulas in the fractured and adjacent vertebrae, which function as internal fixators; a lordotic moment is applied via the cannulas, allowing reduction of the lordosis while the fractured vertebra is simultaneously filled with cement.
Vertebral body remodeling devices all differ from vertebroplasty, lordoplasty, or kyphoplasty as they involve the permanent implantation within the vertebral body of a device in addition to bone cement. Whether implantable devices have greater effectiveness and safety over vertebroplasty and kyphoplasty is not known at this time.
The Kiva™ device (Benvenue Medical Inc, Santa Clara, CA) is a polyether ether ketone (peek) implant (PEEK-OPTIMA®) which is advanced via a transpedicular approach through a Nitinol (nickel titanium) Kiva wire (Fig. 5). This device is currently being investigated in a multicenter trial, the KAST Study (Kiva™ System as a Vertebral Augmentation Treatment—A Safety and Effectiveness Trial).
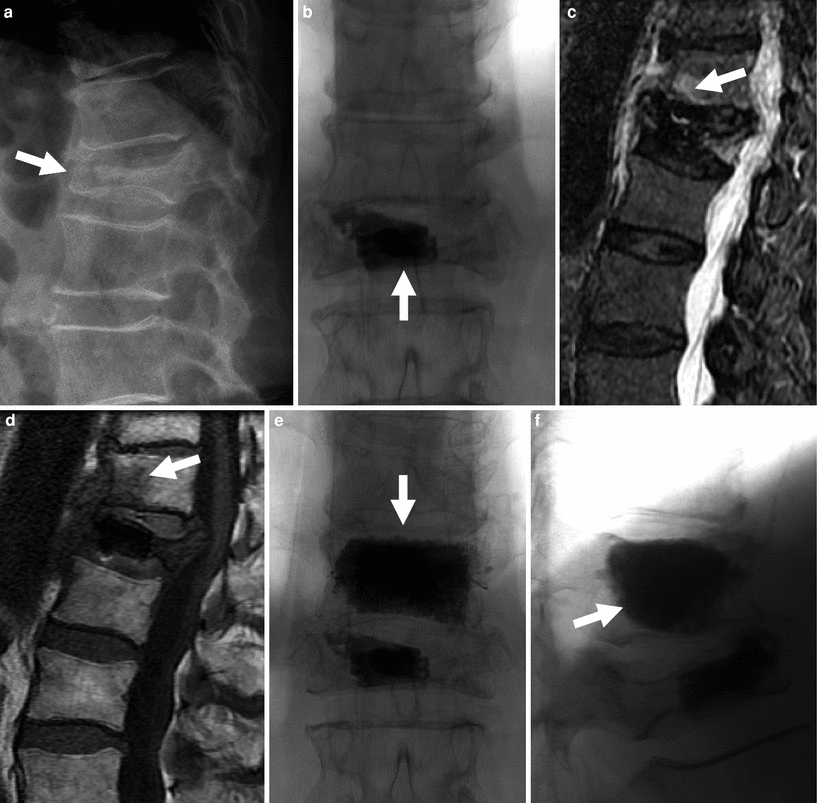

Fig. 5
72-year-old woman with painful L1 fracture treated with the Kiva™ device. Recurrent pain is caused by a fracture at T12, subsequently treated with vertebroplasty. a Thoraco-lumbar spine X-ray shows a fracture at L1 with 70 % height loss and moderate retropulsion (arrow). b Antero-posterior view of the lumbar spine shows the Kiva device and a small amount of bone cement within the vertebral body of L1 (arrow). T2-weighted (c) and T1-weighted (d) MRI shows bone marrow edema in fractured T12 vertebral body (arrows). Following vertebroplasty, AP (e) and lateral views (f) show diffuse and even filling of the T12 vertebral body (arrows). Pain relief was immediate
The StaXx FX Structural Kyphoplasty System® (Spine Wave, Shelton, CT) is another remodeling device which consists of wafers implanted in the fractured vertebra via a percutaneous peripedicular approach and a wide-based inserting needle. StaXx wafers are made from polyether ether ketone and are 1 mm thick each. Wafers are inserted one at a time, using a wedge action to create vertical lift and reduce the fractured vertebral body. The first wafer, or base wafer, acts as a foundation for subsequently inserted wafers. Once all wafers are inserted, bone cement is injected into the vertebral body for further fixation and stabilization. A small volume of cement is also specifically injected anteriorly at the base of the wafer stack, securing the anterior column.
The Optimesh® device (Spineology, Saint-Paul, MN) is a surgical mesh made of polyethylene terephthalate (PET). The mesh pouch, which contains impacted granular bone graft, is inserted in its empty state through a small cannula and then packed in situ with bone graft once in place. As more bone graft material is added to the mesh, the gradually increasing volume deploys the OptiMesh® implant in its final geometric state and generates significant distractive force. When completely filled, the OptiMesh® implant fibers become taut and granular mechanics transforms the contained graft into a custom-fit, rigid, load-bearing graft pack. The Optimesh® device is radiolucent and compatible with all imaging modalities.
The VerteLift™ implant (SpineAlign Medical Inc, Pleasanton, CA) is a wire made of Nitinol alloy which comes in two basic shapes and a range of sizes. The device acts as an internal scaffold to engage the vertebral body endplates, while providing and maintaining lift until bone cement is injected. Prior to injection of bone cement, the VerteLift™ implant is fully retrievable. The VerteLift™ implant is currently approved in Europe, and undergoing investigational device exemption evaluation in the United States.
4 Indications and Contraindications
Currently accepted indications include painful vertebral compression fractures from (a) osteoporosis (primary or secondary), (b) neoplastic infiltration, (c) painful, “aggressive” vertebral hemangiomas, and (d) trauma when minimal displacement is present and surgery is contraindicated (Fig. 3).
There are no absolute contraindications to vertebroplasty or other vertebral augmentation procedures. There are relative contraindications to vertebral augmentation procedures that include (1) the presence of a systemic infection, and (2) lack of appropriate surgical backup, which could delay treatment. Bleeding conditions are not considered a contraindication, as they can be adequately controlled in the majority of patients prior to the procedure.
5 Complications
All complications of vertebral augmentation procedures are relatively uncommon, particularly severe ones. In the early 1990s, the United States FDA initiated the Manufacturer and User Facility Device Experience (MAUDE), a nationwide database which was designed to record the details of medical complications occurring from the use of medical devices associated with indexed procedures. Recorded data consists of user facility reports from 1991, distributor reports from 1993, voluntary reports from June 1993, and manufacturer reports from August 1996.
The earliest reports concerning vertebroplasty and kyphoplasty original clinical reports were filed in 1999. In November 2004, the first FDA MAUDE report on complications resulting from the use of medical devices associated with vertebroplasty and kyphoplasty was published.
A total of 43 adverse events were reported out of approximately 190,000 procedures (0.02 %). Reported complications included 4 deaths, 21 instances of neurologic deficits related to cement canal intrusion or epidural hematoma (6 of which were permanent), 3 episodes of blood pressure drop, 2 pulmonary embolisms, 2 infections (1 diskitis, 1 osteomyelitis), 1 pneumothorax, and 11 technical reports of inconsequential equipment breakage. Twenty-five of the 43 events were major, including 4 deaths and 21 cord compressions requiring surgery, with 6 permanent neurologic injuries (Nussbaum et al. 2004). These data, however, likely underrepresent the actual complication rate from these procedures which is better reflected in clinical studies. Deaths were presumed and reported to occur as a result of reactions to the acrylic bone cement, the free polymer portion of which has known cardiotoxicity and can cause cardiac arrhythmias and hemodynamic instability (Kaufmann et al. 2002). As the risk is dose-dependent, this complication has only been reported when a large number of vertebrae were treated per session.
Neurologic compromise can occur from spinal cord compression because of leakage of large amounts of cement into the epidural venous plexus (Shapiro et al. 2003; Lee et al. 2002; Harrington 2001), requiring expedited surgical evacuation (Shapiro et al. 2003). Cement leakage may also cause direct nerve root compression which can cause new pain or exacerbation of pain (Lee et al. 2002).
Leaking cement in the paravertebral space surrounding the vertebral body usually does not lead to clinical complications, and may occur in as many as 10 % of procedures, (Coumans et al. 2003; Mathis et al. 2001) although transient dysphagia has been specifically reported at the cervical level from esophageal compression (Depriester et al. 1999). Leakage of cement into the intervertebral disk, especially in osteoporotic fractures with rupture of the inferior vertebral body endplate, may occur, without reported clinical consequences (Depriester et al. 1999). With vertebral puncture, there is also a risk of fracture, avoided by meticulous positioning with directed fluoroscopic technique (Pierot and Boulin 1999) or CT guidance in selected instances (Gangi et al. 1998).
With a posterolateral approach (Laredo et al. 1994), there is a risk of pneumothorax at the thoracic level, and of psoas hematoma at the lumbar level (Table 1).
Table 1
Complications of vertebral augmentation procedures
Severe |
Canal intrusion/epidural hematoma with permanent neurological damage |
Infection (diskitis, osteomyelitis) |
Pulmonary embolism |
Myocardial infarction |
Death |
Moderate |
Canal intrusion/epidural hematoma with transient neurological damage |
Reactions to the acrylic (polymethylmethacrylate) bone cement – > cardiotoxicity, cardiac arrhythmias, and hemodynamic instability |
Pneumothorax |
Intraprocedural blood pressure drop |
Minor |
Uneventful equipment failure |
Rib fractures |
Transient post-kyphoplasty radicular pain |
6 Specific Issues with the Geriatric Population
In the elderly, a number of specific issues require special attention, including pre-treatment work-up, procedural technique, and follow-up.
6.1 Adequate Identification of Fractures
Compression fractures have been traditionally diagnosed on plain radiographs, which allow evaluation of bone structure, including the posterior vertebral body wall, and quantification of height loss when present. In the elderly population, several fractures of various ages may coexist, which can complicate identification of the symptomatic level on X-ray imaging alone, even if combined with fluoroscopic-guided provocative manual palpation and a reliable clinical examination. The age of a fracture is an important determinant of response to treatment, and plays an important role in treatment option selection, i.e. in considering the potential superiority of kyphoplasty or other augmentation procedures over vertebroplasty (Spiegl et al. 2009). Magnetic resonance imaging (MRI) is the mainstay of patient evaluation. It is very useful in dating a fracture, showing bone marrow edema in the early stages of a fracture that is not present in older fractures (Fig. 6) (Do 2000; Lindsay et al. 2001). In particular for patients with multiple myeloma, MRI has been shown to be superior to bone scintigraphy (Masala et al. 2005).
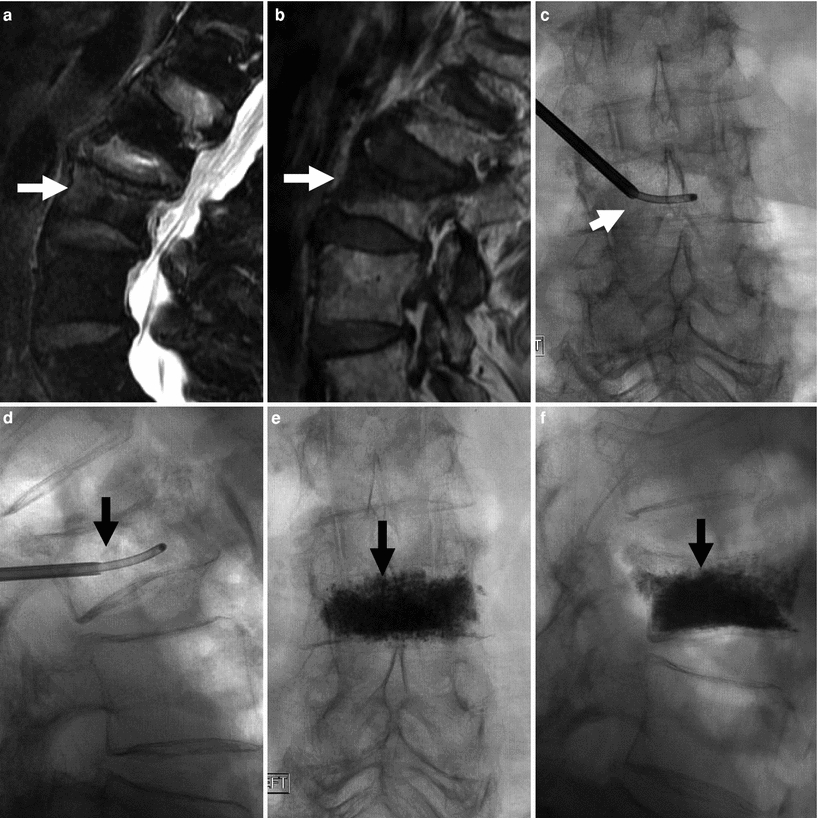

Fig. 6
86-year-old woman with very painful L3 fracture. Unilateral transpedicular vertebroplasty using an 11G curved needle. MRI, T2 (a), and T1 (b) weighted sagittal imaging shows diffused edema in the superior and anterior vertebral body of L3 (arrows). X-rays AP (c) and lateral (d) views show placement of the curved needle within the vertebral body (arrows). X-ray AP (e) and lateral (f) views show cement diffusely and evenly filling the L3 vertebral body (arrows), resulting in excellent pain relief
The work-up of metastatic spinal lesions also heavily relies on MRI which allows objective and reproducible quantitative assessment of the degree of compression, epidural extension, paraspinal extension, presence of other lesions, and the degree of vascularity (Georgy 2008).
A special mention must be made of SPECT/CT (single photon emission computed tomography/X-ray computed tomography), a relatively new hybrid application which combines metabolic information from SPECT images with accurate anatomical information from CT. This technique may be particularly useful in older patients with multiple fractures and severe claustrophobia, pacemaker, or other contraindication to MRI (Suárez et al 2009; Sudhakar et al. 2010) (Fig. 7).
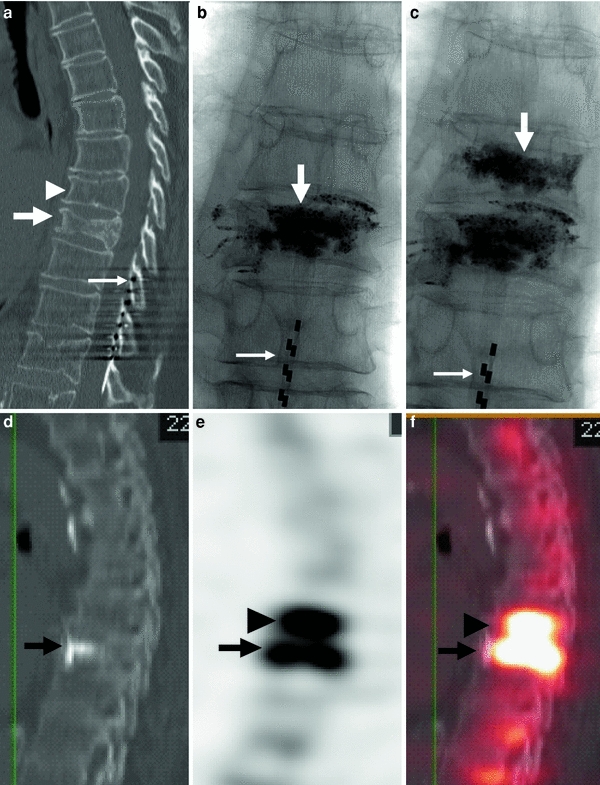

Fig. 7
80-year-old woman with atrial fibrillation and a pacemaker. Severe back pain is evaluated by CT which shows a fracture of the T8 vertebral body. Severe residual pain is assessed with SPECT/CT which shows a fracture at T7. Repeat vertebroplasty at this level results in pain relief. a CT of the thoracic spine, sagittal reconstruction shows a fracture of T8 (arrow). Mild irregularity of T7 is also present (arrowhead). Note spinal cord stimulator (thin long arrow). b X-ray of the thoracic spine, AP view, shows cement in the T8 vertebral body. Note spinal cord stimulator (thin long arrow). c X-ray of the thoracic spine, AP view, shows cement in T7 and T8. Note spinal cord stimulator (thin long arrow). d, e, f SPECT/CT shows significant uptake of Tc-199 m not only in the vertebral body of recently treated T8 (arrow), but also in the T7 vertebral body (arrowhead)
6.2 Analgesia
Elderly patients who present for the evaluation and treatment of a vertebral compression fracture are often on narcotic pain medications for chronic pain from various causes, and their medication dependency may be exacerbated by the presence of a compression fracture. These patients commonly have a higher response threshold than average, for which higher doses of sedation are typically required during a procedure, and often they suffer from some degree of confusion. In anticipation of a procedure in such patients, it is helpful to reduce the oral intake of narcotics and attempt substituting anti-inflammatory drugs, i.e. ibuprofen or ketorolac (Toradol®), to better control intraprocedural sedation.
6.3 Patient Positioning
Elderly patients have a high incidence of spondylosis, arthritis, and advanced osteoporosis.
Careful and methodical positioning is particularly important in order to avoid causing new pathology, especially as osteoporosis results in challenging fluoroscopic visualization of bony structures. Rib fractures may occur relatively easily in these patients as a result of suboptimal positioning on the X-ray table, or from pressure on the ribcage from needle insertion through the pedicles. Particular attention should be directed to supplemental padding of contact points. Muscle spasms may also appear after such procedures and can be exacerbated by positioning maneuvers. Patients with a high level of confusion may be at risk for falling off the fluoroscopic table and therefore need to be closely monitored.
6.4 Procedural Sedation
Elderly patients may have significant age-related decreases in drug clearance, resulting in higher bioavailability of narcotic or other drugs taken at home prior to a procedure. In addition, renal and hepatic clearance of intravenous drugs may be significantly prolonged from age-related diminished enzyme activity, and in this patient population these complicating factors may not be accurately predicted from serum levels of creatinine and liver function tests. Particular care must be taken when midazolam is used for sedation, as a sudden drop in oxygenation may occur: severe drops in oxygen saturation levels may require emergent administration of a reversal agent. It is generally advisable to use as little sedation as possible in these patients, which emphasizes again the need for adequate patient preparation and education prior to the procedure (Luginbühl 2008).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree