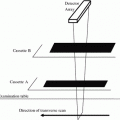
Fig. 1
Description of BUA where (a) the amplitude spectra for a sample and reference material are compared resulting in (b) attenuation as a function of frequency. Broadband ultrasound attenuation (BUA) is the slope of the regression line
3.3 Amplitude Dependent-Speed of Sound, Stiffness Index and Quantitative Ultrasound Index
Both SoS and BUA are influenced by the coupling medium (temperature of water, type of gel) and by the properties of soft tissue layers like temperature and/or edema (Barkmann and Glüer 1999; Johansen and Stone 1997).
As a consequence, more complex parameters like Amplitude Dependent-Speed of Sound (AD-SoS), Stiffness Index (SI), and Quantitative Ultrasound Index (QUI) have been developed to obtain higher precision and less pronounced sensitivity to external influencing factors (Gluer 1997).
These new parameters have proved to be useful not only in the identification of subjects with low bone mineral density and at high risk for fracture (Hans et al. 1996; Guglielmi et al. 2003), but also, and above all, in the investigation of the significant alterations in bone elasticity and structure in metabolic pathologies affecting the skeleton. This new approach to the interaction between ultrasound and bone tissue has led to the availability of complementary data to those provided by densitometry techniques (Roben et al. 2001; Zitmann et al. 2002; Passeri et al. 2003).
4 QUS Measurement Sites and Approaches
The most common skeletal sites currently studied by QUS techniques are the calcaneus, the distal metaphysis of the phalanx, the radius, and the tibia.
4.1 Calcaneus
In 1984 Langton, in his first pioneering model (Langton et al. 1984), suggested transverse transmission in cancellous bone basing on the principle that the site of measurement and the transducers had to be immersed in a temperature controlled water bath to ensure proper coupling of the acoustic wave into the skin.
The most preferred site become the calcaneus, which is made up almost entirely of trabecular bone and has the advantage of featuring two flat, parallel surfaces that reduce repositioning errors and are very useful for optimizing the geometry of transmission of the ultrasound band through it.
Therefore a variety of devices measuring ultrasound transmission through the human calcaneus have been developed with several differences regarding to the coupling of the ultrasound wave into the body and the selection of the region to be measured.
Recent Achilles (GE Healthcare, Madison, USA) models, which use water encapsulated in compliant membranes that conform closely to the patient’s foot through a temperature-controlled medium, have left the UBIS 5000 (Diagnostic Medical Systems, Pérois, France) and the DTU-One (Osteometer MediTech, Hawthorne, USA) as the main water-bath systems actually on the market.
In fact the greater part of manufacturers adopted the gel as a coupling medium in order to avoid water reservoirs. A proper coupling into the skin has been reached with the use of transducers which are pressed against the skin, usually with the help of rubber pads (CUBA Clinical, McCue—Sahara, Hologic, Bedford, USA).
Several in vitro studies demonstrated that within the same measurement site (i.e. the calcaneus) there is a pronounced variation in BUA and SOS depending upon the localization of the region of interest (Damilakis et al. 2000).
The importance to avoid measurement errors due to variable positioning caused by different foot sizes led to the introduction of several techniques capable to obtain an image of the calcaneus.
An adjustable region of interest (ROI) shown on the display lets the operator confirm the approximate location of where the ultrasound beam will enter the heel during measurement, ensuring optimal positioning and increasing confidence in measurement.
Although numerous devices for QUS measurement of calcaneus are available on the market, few of these have attained an acceptable level of scientific validation in the prediction of fracture risk.
4.2 Phalanx
The metaphysis of the phalanx is consisting of trabecular (at about 40 %) and cortical component, the main determinant of biomechanical bone strength (Fig. 2).
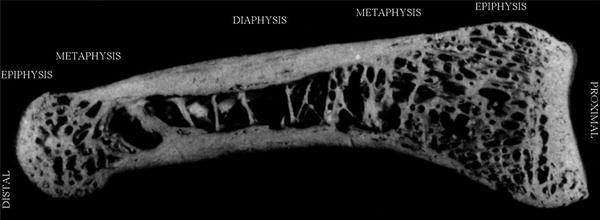

Fig. 2
Picture of human phalanx showing the trabecular and cortical component
Transverse transmission of cortical bone has been chosen at that level for its high bone turnover and for the sensitivity to changes regarding the skeleton: growth and aging (natural causes), metabolic diseases (hyperparathyroidism…) or drugs (glucocorticoids) (Montagnani et al. 2002) (Fig. 3).
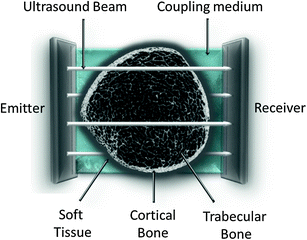

Fig. 3
Transversal ultrasound transmission
The DBM Sonic Bone Profiler (Igea, Carpi, Italy), unique device that uses this method, measures proximal finger phalanges from II–V by a hand-held caliper with two transducers positioned on opposite sites of the metaphysis and acting as transmitter and receiver respectively.
4.3 Tibia, Radius and Other Sites
Axial transmission of ultrasound through tibia and radius is actually available in only one device (Omnisense, BeamMed-Sunlight Ultrasound Technologies, Rehovot, Israel). Unlike all the other products, the one side approach offers the potential to measure a variety of bones (metacarpus, humerus, and others), even if tibia and radius remain the most validated skeletal sites demonstrating a good sensitivity to endosteal reabsorption phenomena (Barkmann et al. 2000a). Since propagation occurs mainly along the external surface of the bone, this technique provides indications mostly on the intrinsic material properties of cortical bone (Raum et al. 2005) (Fig. 4).
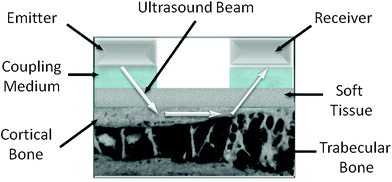

Fig. 4
Axial ultrasound transmission
Other skeletal sites like femur and spine have been suggested for evaluation of bone tissue for the assessment of axial BMD at the most common and important sites of fragility fractures.
5 Quality Assurance
The quantitative study of bone tissue requires an adequate reproducibility in terms of reproducibility of measurements performed by different operators with the same medical device and the reproducibility of the measurement performed with different devices of the same model.
First of all a comprehensive training program for the staff is necessary because the apparent fast and easy method of assessing bone status can give the false impression that measurement is always adequately performed. On the contrary, the absence of an immediate feedback on the quality of examination (as it is for DXA) requires a careful adherence to protocol by the operator.
Second, to limit all possible sources of errors, it is fundamental to strictly follow the quality assurance procedures developed for QUS devices.
A constant check of the calibration of the apparatus is mandatory to ensure correct emission of the ultrasound signal by the transducers (i.e., a check of correct functioning of the probes) and the determination of the speed of propagation of the impulse emitted. All devices on the market usually require routine testing for probes and calibration with specific phantoms (usually composed of plastic material or of plexiglass) and own procedures capable of recognize and signal any kind of malfunction problems (Thijssen et al. 2007).
Since QUS devices are portable, environmental conditions become extremely important particularly for calcaneus devices that use water as the coupling medium between transducers. The dependence of the measurement on variations in the temperature of the water is more evident with the use of phantom and has been well-observed by several authors (Chappard et al. 1999; Barkmann et al. 1996; Paggiosi et al. 2005; Krieg et al. 2002; Ikeda and Iki 2004; Mentzel et al. 2009).
Various longitudinal studies attempted to establish quality control for the calcaneus devices proposing a combination of external phantom and internal indicators based on the water measurements (Barkmann and Gluer 1998). Langton (Langton 1997) proposed an external electronic phantom in order to reach a better stability and to reduce the influence of external factors while Laugier et al. (1996) suggested the use of internal digital phantoms; this system has been adopted by the UBIS 5000 (Diagnostic Medical Systems, Pérois, France) showing encouraging results (Hans et al. 2005) but the overall complexity of these quality assurance procedures still results poorly suitable for clinical practice (Hans et al. 2002; Chen et al. 2012).
The issue of cross calibration between different QUS devices assumes determining importance not only in case of equipment change in a single clinic, but most of all in multicenter clinical studies where data are to be collected in different places with several devices, even from the same manufacturer. In the osteoporosis and ultrasound (OPUS) European multicenter clinical study Gluer and colleagues (2004) proposed a single specific phantom for all the devices and a monitor subject who underwent periodic tests at all the centers involved in the study with the aim to check the agreement and calculate the error of reproducibility among the devices.
Although several progresses have been achieved in this field, at the moment there are not yet adequate procedures for standardizing QUS devices, even in those that perform measurements on the same skeletal site.
An important point of discussion is related to the population-based geographic variations in bone density in Europe. Although the OPUS study, the Network in Europe on Male Osteoporosis (NEMO) study (Kaptoge et al. 2008) and the European Vertebral Osteoporosis (EVOS) study (Lunt et al. 1997) have shown between-center differences in hip bone mineral density in European women, an appropriate knowledge on the international geographical heterogeneity of QUS measurement variables has not been reached yet (Paggiosi et al. 2011).
Recently, Pye et al. (2010) described significant between–center differences in BUA, SOS, and QUI in the European Male Aging Study (EMAS) with no consistent geographical trends in results between North-western, Southern or Eastern Europe. However data have been acquired with a single manufacturer type of device (Sahara, Hologic, USA). In order to avoid these limitations, Paggiosi and colleagues (2012) have recently examined the data acquired with a different range of QUS devices during the OPUS study. The aim was to demonstrate a significant geographical variation in QUS measurement variables, to compare them with hip BMD and to analyze the influence of anthropometric characteristics on the heterogeneity of QUS parameters. The results suggested that QUS measurement variables vary between European countries in a different way to those for hip BMD. These variations may be explained by the influence of anthropometric characteristics, the heterogeneity of QUS devices and other factors that still remain difficult to assess and quantify.
Although a validated cross-calibration procedure still remains elusive, a statistical approach to achieve a standardization of data can be adopted to reduce any between–center differences in QUS measurement variables. However, a widely application of this method still needs further validation.
6 Experience In Vitro and In Vivo
6.1 Ultrasonic Propagation Models
A detailed understanding of ultrasound parameters and their relations with bone characteristics is a key to the development of bone QUS.
Historical models of ultrasonic propagation revealed the importance of both the viscous effects of bone marrow and the anisotropy of the porous microstructure.
Considering the elastic modulus of the cancellous framework as a major determinant of ultrasound speed, Biot developed a wide applicable theory that predicts wave propagation in a two phase heterogeneous media (fluid saturated porous solid). First Taking into account the motion of the fluid and the solid independently and second the viscoelastic coupling between them, two longitudinal waves, fast wave and slow wave are observed. The fast wave moves within the mineralized materials and the slower one moves through the inter trabecular medullar structure (McKelvie and Palmer 1991).
However in practice it has been notified that, due to superimposition phenomena, it is difficult to identify these waves in the received signal and the extraction of information can be difficult to achieve. To obtain a proper separation of the waves Grimes et al. used a space alternating generalized expectation maximization (SAGE) algorithm and got parameters such as arrival time, center frequency, bandwidth, amplitude, phase and velocity of each wave (Grimes et al. 2012).
In these years other important limitations of classical wave propagation theories have been notified (Leclaire et al. 1997). These considerations have induced the development of several studies employing wave equations as alternative formulation of Biot’s theory (Gautier et al. 2011; Hughes et al. 2007) or other numerical modeling methods (Haïat et al. 2007; Nelson et al. 2011; Lawrence et al. 2010).
Although the results seem to be promising, no single model is actually capable to explain the acoustic behavior of bone; future developments should consider additional attenuation mechanisms as scattering, local flow in micro-cracks and surface roughness of the trabeculae in a fully comprehensive theory (Haire et al. 1999).
6.2 Cancellous Bone
Although the influence on speed of sound of both mineral content and porosity still remains to be deeply elucidated, several in vivo clinical trials and in vitro laboratory measurements have confirmed a good correlation between SoS and density (r2 0.78–0.91) (de Terlizzi et al. 2000). On the other hand BUA seems to have a stronger correlation with trabecular porosity and can be a useful indicator of bone structural changes (Gluer et al. 2004; Tavakoli and Evans 1991, 1992).
It has been observed that SoS are strongly dependent on the orientation of the measurement in the same skeletal site (Strelitzki et al. 1997). This element suggested that, even though is greatly influenced by the mineral density, SoS reflects other properties as the degree of alignment of the trabecular structure and the elastic modulus of bone (Yamamoto et al. 2012; Hans et al. 1999; Wear et al. 2012; Grondin et al. 2012). Nicholson and Bouxsein (2000) studied human calcaneus bones to evaluate QUS variables before and after the compression of the specimens without finding differences between elastic modulus and measurements. However it has been observed that SoS reflects bone density independently of the bone elastic constants and that the slope of the BUA versus frequency reflects bone elastic constants independently of bone density (Grimm and Williams 1997). Other analysis of different models of trabecular bone demonstrated a better correlation of the SoS with bone volume rather than other structural or elastic components, whereas the BUA appeared to be more influenced by scattering and viscoelastic mechanisms (Sasso et al. 2008).
Other studies showed that SoS and BUA seem not to be capable to provide different and/or complementary characteristics of the bone tissue from those obtained with densitometry techniques.
6.3 Cortical Bone
The bone architecture of the phalanx can be described with a micromechanical model of cortical bone made of two-phase composite material consisting of an anisotropic mineralized matrix pervaded by cylindrical pores. This model influences the characteristics of the ultrasound wave that have propagated through it: the SoS, the shape (number of peaks) and the amplitude of the ultrasound signal (fast wave amplitude) (Cadossi and Canè 1996).
Wuster and colleagues (2005), in a study performed on human phalanges of cadavers analyzed by bone ultrasound, dual-energy X-ray absorptiometry (DXA), and micro quantitative computed tomography, showed a close correlation of SoS and fast wave amplitude with the mineralized spaces of the trabecular and cortical structure. The frequency content of the signal, calculated by Fourier analysis, has been correlated with the inter trabecular spaces occupied by the marrow and the organic matrix.
In a clinical study on human phalanges, Barkmann and colleagues demonstrated that the duration (microseconds) of the fast ultrasound signal and the ADSoS are able to reveal endosteal bone absorption and are correlated with the cortical area and strength (Barkmann et al. 2000b).
Grondin and collegues (2012) investigated the relative contributions of porosity and mineralized matrix properties to the bulk compressional wave velocity (BCV) along the long bone axis and confirmed the hypothesis that the intracortical porosity strongly influences the mesoscopic elasticity (Granke et al. 2011).
Recent studies on excised human radii suggested a sophisticated multimodal approach with several propagating models in order to collect different elements on bone strength and to achieve a more complete status of cortical bone (Muller et al. 2008).
Other authors (Sievänen et al. 2001; Tatarinov et al. 2005; Njeh et al. 1999b) have found an independent contribution to SoS of cortical thickness and cortical BMD at different sites as radius (Bosisio et al. 2007; Muller et al. 2005; Bossy et al. 2004) and tibia (Prevrhal et al. 2001; Tatarinov et al. 2011; Määttä et al. 2009).
6.4 Future Approaches
In the past years a growing attention to new QUS methods of investigation at femur and spinal level have been developed as a response to the lack of direct measurements in the most common sites of fragility fractures (Barkmann et al. 2007; Dencks et al. 2007; Serra-Hsu et al. 2011; Nicholson and Alkalay 2007). Studies in vitro in samples of human femur have shown a high correlation between QUS measurement and bone mineral density (BMD) with a good level of accuracy (Grondin et al. 2010; Bossy et al. 2007; Zebaze et al. 2010).
Machado et al. proposed the evaluation of fracture healing measuring the time of flight (TOF) of ultrasound wave by axial transmission at cortical bovine femur sample. The results have shown that SoS was affected by local changes in mineralization and therefore is sensitive to callus changes during the regeneration process (Machado et al. 2011).
Nauleau and collegues focused their attention to the characterization of thickness and elastic properties of femur applying an algorithm to measure the wavenumbers of several circumferential guided modes in a cylindrical cortical bone-mimicking phantom. The results open interesting perspectives for the ultrasonic characterization of one of the most important site of osteoporosis fracture: the femoral neck (Nauleau et al. 2012).
7 Clinical Experience: Fracture Risk and Further Applications
7.1 Primary Osteoporosis and Fracture Risk
At the beginning the clinical interest in quantitative bone ultrasound centered mainly on the problem of diagnosing osteoporosis. In fact several studies evaluated the performance of ultrasound devices and their ability to discriminate subjects with osteoporotic fractures, especially in the elderly female population and it has been widely demonstrated how the correlation between ultrasound and densitometry values is statistically significant. However, the lack of ultrasound measurement at axial BMD (rachis or femur) and the QUS capability to provide some information about bone quality without ionizing radiation have shifted the attention to the assessment of fracture risk.
In recent years, numerous prospective prospective studies of great importance have been performed to assess fracture risk by QUS at the calcaneus (Bauer et al. 1997; Hans et al. 1996; Krieg et al. 2006) showing a significant association between heel QUS and fracture prediction. One of the latest, the EPIC-Norfolk prospective population study, has been conducted on a British male and female population of 14,824 subjects in an age range of 42– 82 years, with a mean follow-up of 1.9 (0.7) years and definitely proved the efficacy of QUS at the calcaneus in predicting fracture risk (Khaw et al. 2004).
A European cross-sectional multicentre study (phalangeal osteoporosis study PhOS) performed on over 10,000 women provided important confirmation and clinical validation of the QUS method at the phalanx. It demonstrated the high precision (coefficient of variation CV below 1 % in both the short- and long -term) of QUS performed at phalanx and the ability to detect osteoporotic subjects with vertebral or hip fractures (Wuster et al. 2000).
Guglielmi and colleagues compared QUS at the phalanges with X-ray methods (DXA and QCT) and morphometric analysis of hand radiographs. Adopting receiver operating characteristic (ROC) analysis, no differences between the two techniques have been found (Guglielmi et al. 2003). Similar studies conducted by other authors, have also shown similar results (Boonen et al. 2005).
In the Basel Osteoporosis Study (BOS) for detecting vertebral fractures, Hartl and colleagues have shown that QUS at the calcaneus and phalanx has comparable performances with the results obtained with axial DXA (Hartl et al. 2002).
Krieg and colleagues performed a retrospective and cross-sectional study conducted on an elderly (70–80 years of age) Swiss population assessing the ability of calcaneal and phalangeal QUS in discriminating subjects with hip fracture. Although both methods showed encouraging results, QUS at calcaneus proved more effective in the elderly population (Krieg et al. 2003). To this regard, a small but interesting Italian study has highlighted that QUS at phalanx is more sensitive in discriminating subjects with vertebral fracture immediately post-menopause (Camozzi et al. 2007).
As mentioned before, the OPUS study has shown that QUS at the phalanx and calcaneus is effective in identifying subjects with vertebral fractures in a large European population.
Kanis et al. have identified the criteria for risk assessment based on QUS at the phalanx and age and published tables for calculating fracture risk at 10 years (Kanis et al. 2005).
Recently, a large cross-sectional population-based survey called European Male Ageing Study (EMAS) tried to demonstrate the influence of sex hormones on markers of bone turnover and to explore the association between these markers and bone health in middle-aged and elderly European men (Boonen et al. 2011). The results have shown a suggestive evidence of association between a single nucleotide polymorphism with SoS and BUA measured at the calcaneus.
The International Society for Clinical Densitometry (ISCD) has recently published the new position of the society regarding QUS outlining a high level of evidence of heel QUS in osteoporotic fracture prediction. However, as reported by Hans and Krieg in a review article, although several QUS models are available on the market, especially for measurement at the calcaneus, only a small minority of these have a scientific validity confirmed by clinical studies published in literature. Furthermore, the level of evidence in the assessment of osteoporotic fracture risk can be quite different for each device and has been validated in some, but not all populations (Table 1).
Table 1
Evidences in fracture risk assessment for available QUS devices
Manufacturer | Model | Site of measurement | Ability to assess hip fracture risk | Ability to assess spine fracture risk |
|---|---|---|---|---|
GE-medical | Achilles | Calcaneus | Proven in most populations | Proven in most populations |
DMS | UBIS 3000/5000 | Calcaneus | Some evidence | Some evidence |
Hologic | Sahara | Calcaneus | Proven in Caucasian | Proven in Caucasian |
McCue-Norland | Cuba clinical | Calcaneus | Proven in Caucasian | Some evidence |
IGEA | DBM sonic BP | Phalanx | Proven in Caucasian | Proven in Caucasian |
BeamMed | Omnisense | Radius, Tibia | Some evidence | Some evidence |
Meditech | DTU-One | Calcaneus | No evidence | Some evidence |
Aloka | AOS-100 | Calcaneus | Some evidence | No evidence |
Elk Co | CM-100/200 | Calcaneus | No evidence |
Considering these aspects, QUS cannot be claimed to replace densitometry, but rather combines with it; specific algorithms combining instrumental (DXA or QUS) and clinical risk factors should be used to identify women to be treated to avoid future fracture and pathologic ultrasound values must be considered an independent factor of fracture risk.
Several European scientific societies have included QUS in their national guidelines for the assessment of fracure risk for post-menopausal osteoporosis in women who are in the perspective of treatment (National Osteoporosis Society 2002; Schattauer GmbH 2006). In Italy, “note 79” regarding the prescription of anti-osteoporosis drugs has included QUS in the model for estimating fracture risk at 10 years, using the combination of clinical risk factors and T-score values (Agenzia Italiana Del Farmaco 2007).
7.2 Secondary Osteoporosis
The studies of QUS applied in causes of secondary osteoporosis have proved to be useful in characterization of metabolic bone pathologies such as: osteoporosis induced by glucocorticoids (Gonnelli et al. 2010), hyperparathyroidism (Gonnelli et al. 2000), osteomalacia (Luisetto et al. 2000), thalassemia (Filosa and de Terlizzi 2002), osteogenesis imperfecta (Kutilek and Bayer 2010), rheumatoid arthritis (Cryer et al. 2007), psoriatic arthritis (Frediani et al. 2003), epilepsy (Pluskiewicz and Nowakowska 1997), and cystic fibrosis (Rossini et al. 2007; Lopez-Rodriguez et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree