FOLLICLE-STIMULATING HORMONE (FSH)
Action: influence on (1) number of follicles, (2) development of ovarian cysts, (3) size of ovaries
Physiology: rises abruptly at birth ← decrease in estrogen + progesterone after loss of placenta; falls to low levels after 3 months of age; remains low until puberty
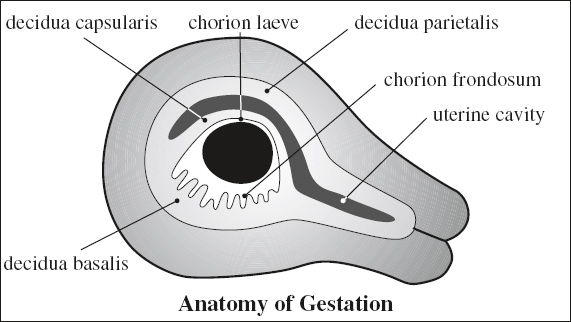
ANATOMY OF GESTATION
Choriodecidua
Chorion
[chorion, Greek = skin, leather]
= trophoblast + fetal mesenchyme with villous stems protruding into decidua; provides nutrition for developing embryo
(a) chorion frondosum = part adjacent to decidua basalis, forms primordial placenta
[ frondosus , Latin = foliage]
(b) chorion laeve = smooth portion of chorion with atrophied villi
(c) “chorionic plate” = amnionic membrane covering the chorionic plate of the placenta
Decidua
(a) decidua basalis = between chorion frondosum + myometrium
(b) decidua capsularis = portion protruding into uterine cavity
(c) decidua parietalis = decidua vera = portion lining the uterine cavity elsewhere
Gestational Sac (GS)
Origin: arises from blastocyst, which implants into secretory endometrium 6–9 days after ovulation (= 20–23 days of MA), surrounded by echogenic trophoblast
◊ GS measures 0.1 mm at time of implantation
√ “intradecidual” sign (earliest sign: 48% sensitive, 66% specific, 45% accurate at < 5 weeks GA) = eccentrically located fluid collection corresponding to gestational sac completely embedded within an echogenic decidua adjacent to a thin echogenic line (= collapsed uterine cavity) = highly suggestive for IUP
A nonspecific fluid collection with a smooth spherical / oval contour represents an IUP until proven otherwise!
√ “double (decidual) sac” sign (DDS) [most useful > 5 weeks GA with a mean sac diameter of 10 mm / 40 days GA] = 2 concentric hyperechoic rings surrounding a portion of the gestational sac + bulging into uterine cavity (delineated by a thin crescent of endometrial fluid) = definitive IUP
(a) outer echogenic ring (= decidua parietalis)
(b) inner echogenic ring (= decidua capsularis)
√ GS surrounded by endometrial thickening > 12 mm
√ continuous hyperechoic inner rim > 2 mm thick
√ GS of spherical / ovoid shape without angulations
√ interposed hypoechoic line (apposed endometrial walls)
√ DDS present with a mean sac diameter of 10 mm (= 40 days GA)
◊ A “double decidual sac” sign correlates with the presence of a pregnancy in 98%!
◊ Absence of these 2 signs (in 35%) does not exclude an IUP
Gestational Sac Size
= average of 2–3 diameters (craniocaudad, AP, TRV) of rounded intrauterine anechoic (fluid) space within sac walls
◊ Used for dating between 6 and 12 weeks MA (identified as early as 4.5–5.0 weeks MA on transabdominal scan)
EGA [in wk] = (GS [in mm] + 25.43) ÷ 7.02
Accuracy: ± 7 days
Growth rate of mean sac diameter (MSD):
1.13 (range 0.71–1.75) mm per day (often variable)
| Linear growth: | 10 mm by | 5th | week MA |
| 60 mm by | 12th | week MA |
fills chorionic cavity by 11th–12th week MA
An MSD cutoff of 25 mm WITHOUT an embryo is a criterion for definitive pregnancy failure;
An MSD range of 16–24 mm WITHOUT an embryo is an indicator of suspicion of pregnancy failure.
Visualization of Gestational Sac
Earliest visualization: mean sac diameter of 2–3 mm
A. GS VISUALIZATION VERSUS β-hCG LEVEL (2nd International Standard):
(a) on transabdominal scan:
| in | 100% | with β-hCG levels of | > 1,800 IU/L |
(b) on transvaginal scan:
| in | 20% | with β-hCG levels of | < 500 IU/L |
| in | 80% | with β-hCG levels of | 500–1,000 IU/L |
| in | 100% | with β-hCG levels of | > 1,000 IU/L |
B. GS VISUALIZATION VERSUS MENSTRUAL AGE
| 5.0 ± 1 weeks = | 10 mm |
| 5.5 ± 1 weeks = | 13 mm |
| 6.0 ± 1 weeks = | 17 mm |
| 6.5 ± 1 weeks = | 20 mm |
C. GS VISUALIZATION VERSUS EMBRYO
Secondary Yolk Sac
= rounded sonolucent structure (outside amniotic cavity) within chorionic sac (= extracoelomic cavity) connected to umbilicus via a narrow stalk; formed by proliferation of endodermal cells; part of yolk sac is incorporated into fetal gut; the rest persists as sac connected to fetus by vitelline duct
Function:
(a) transfer of nutrients from trophoblast to embryo prior to functioning placental circulation
(b) early formation of blood vessels + blood precursors on sac wall
(c) formation of primitive gut
(d) source of primordial germ cells
Time of formation: at around 28 days MA
Mean size: 1.0 mm by 4.7 weeks MA; 2.0 mm by 5.6 weeks MA; 3.0 mm by 7.1 weeks MA; 4.0 (2.2–5.3) mm by 10 weeks MA; disappears around 12 weeks MA
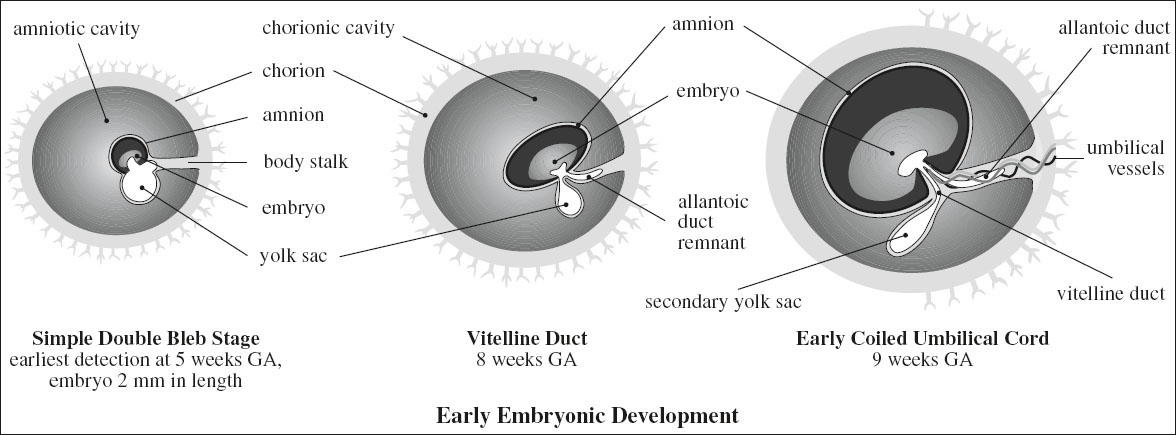
◊ First visible structure within gestational sac = confirms IUP
√ eccentrically located within gestational sac (inside chorionic cavity)
Definite visualization on transvaginal scan:
√ at 5.5 weeks MA
√ inside GS with a mean sac diameter of ≥ 8 mm
Definite visualization on transabdominal scan:
√ at 7 weeks MA
√ inside GS with a mean diameter of ≥ 20 mm
Embryo
Developmental stages:
Preembryonic period: 2nd–4th week MA
Trilaminar embryonic disk: during 5th week MA
Embryonic period: 6th–10th week MA
Physiologic umbilical herniation: 8th–12th week MA
Fetal period: early 11th week MA
Average growth rate:
0.7 mm per day / 1.5 mm every 2 days
√ curvilinear growth from 7 mm at 6.3 weeks MA → to 50 mm at 12.0 weeks MA
Earliest visualization (on endovaginal scan):
at 5.4 weeks MA at CRL of 1.2 mm
√ eccentrically located at periphery of yolk sac (inside amniotic cavity)
◊ Most accurate measurement of GA in 1st trimester!
VISUALIZATION OF EMBRYO VERSUS GS
(a) on transabdominal scan
100% visualization if gestational sac ≥ 27 mm
(b) on transvaginal scan
100% visualization if gestational sac ≥ 12 mm
◊ Transvaginal scan not necessary if on transabdominal scan gestational sac > 27 mm without evidence of embryo!
Failed pregnancy: nonvisualization of embryo with mean gestational sac size of ≥ 18 mm
Cardiac Activity of Embryo
◊ Heart begins to contract at a CRL of 1.5–3.0 mm = 22 days GA = 36 days MA
√ absence of cardiac activity in embryos < 4 mm may be normal
Definite visualization on endovaginal scan:
(a) at 46 days GA
(b) at mean sac diameter of 16 mm
(c) with CRL ≥ 5 mm = 6.2 weeks
A 7-mm CRL is necessary to yield a specificity and positive predictive value of 100% → decreasing false-positive diagnosis associated with a 5-mm CRL cutoff.
Definite visualization on transabdominal scan:
(a) at 55 days GA
(b) mean sac diameter of 25 mm
Heart rate:
at 5-6 weeks GA 101 bpm
at 8-9 weeks GA 143 bpm
Gastrulation (2nd–4th week GA)
[gaster, Latin = stomach; -ula, Latin = diminutive suffix]
= transformation of bilaminar disk into trilaminar embryo
Stages:
› bilaminar embryo develops an epiblast (= layer facing amniotic cavity) + hypoblast (= layer facing yolk sac)
› disk forms a mesoderm “sandwich” bordered above by ectoderm + below by endoderm
› at both ends where embryonic ectoderm and endoderm meet are cranially the oropharyngeal membrane (= future mouth) + caudally the cloacal membrane (= future urogenital and anal orifices)
› craniocaudal + lateral folding converts the flat trilaminar embryonic disk into a gut tube within a body tube
› lateral edges of somatic mesoderm move ventrally toward each other creating a coelomic space (= future peritoneal cavity) that separates gut from body tube (GI tract from primary abdominal wall)
› myoblasts migrate into primary abdominal wall forming muscles and connective tissue
› the cloaca (= common chamber at caudal end) forms as precursor to the intestinal + urinary + genital tracts
› a ventral diverticulum (= allantois) projects from the cloaca into the connecting stalk (= body stalk) attaching the embryo to the chorionic mesoderm
Amnionic Membrane
= curvilinear echogenic line within chorionic sac; fills chorionic cavity by 11–12 weeks MA ← onset of fetal urine production at about 10 weeks
Fusion:
› fuses with chorionic membrane at 14–16 weeks MA to form the chorionic plate
› incomplete fusion with chorion frequent
(DDx: subchorionic hemorrhage, twin abortion, coexistent with limb-body wall complex)
Umbilical Cord
Function: communication between placenta and fetus allowing exchange of gas and nutrients
Embryology:
› cord forms between 7th–8th week post conception with contributions from body stalk, omphalomesenteric or vitelline duct, yolk sac, allantois
› junction of the amnion with ventral surface of embryo forms umbilicus
› allantoic vessels establish continuity with placental villi and form the vessels of the umbilical cord
› allantois / urachus develops as 2nd outpouching from primitive gut 12–16 weeks GA and projects into connecting stalk
› physiologic midgut herniation into base of umbilical cord at ~ 7–12 weeks (returns by end of 11th week GA)
› cord grows until end of 2nd trimester: average diameter of 17 mm, length of 50–60 cm
Anatomy:
› 2 umbilical arteries (= branches of the 2 internal iliac arteries) + 2 umbilical veins by 6 weeks GA
Function: deoxygenated blood from fetus to placenta
› one umbilical vein (remains after regression of right umbilical vein by 8 weeks GA) → connects to left portal vein → fissure for ligamentum venosum after birth
Function: oxygenated blood from placenta to fetus
› covered with unique compressible matrix of mucopolysaccharide-rich substance = Wharton jelly
› covered by amnion
› spiraling of cord with 0–40 helical turns (L > R) by 9 weeks
◊ coiling adds strength + resists compression of vessels
◊ normal cord length + coiling requires adequate fluid space and fetal activity
Cord thickness: 1–2 cm in diameter
(a) “lean” umbilical cord = cross-sectional area of cord measuring below 10th percentile for GA
Associated with: increased prevalence of fetal IUGR, oligohydramnios, fetal distress during delivery
(b) “fat” umbilical cord
Associated with: mothers with diabetes, fetus with aneuploidy
Umbilical coiling index (UCI):
= number of cord spirals completed per cm of cord length
(a) hypocoiled cord (UCI ≤ 0.29 / < 10th percentile)
Associated with:
increased rate of IUGR, fetal demise, intrapartum fetal heart rate decelerations, fetal distress at delivery, karyotype abnormalities, higher rate of abnormal cord insertion
(b) hypercoiled cord (UCI ≥ 0.6 / > 90th percentile)
Associated with:
increased rate of fetal IUGR, intrapartum fetal cardiac decelerations, vascular thrombosis, cord stenosis
PLACENTA
[ plakuos , Greek = flat cake]
(a) fetal portion
1. Villi of chorion frondosum
contain arterial plexuses supplied by umbilical artery and protrude into intervillous space bathing in maternal blood
(b) maternal portion
2. Decidua placentalis: lines intervillous space
Imaging of Normal Placenta
CT:
@ 1st trimester
√ placenta indistinguishable from myometrium
@ 2nd trimester
√ hyperattenuating relative to subjacent myometrium following enhancement
√ rounded hypoattenuating foci surrounded by enhancing placenta = placental cotyledons
@ 3rd trimester
√ increased heterogeneity of normal placenta and visualization of venous lakes
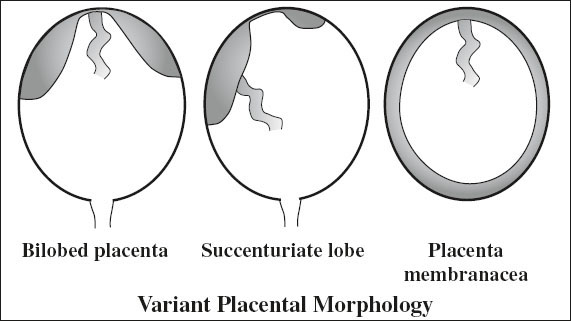
Variant Morphology of Placenta
A. Additional lobes
1. Succenturiate lobe of placenta
= ACCESSORY LOBE
= single / multiple separate additional lobe(s) separate from but connected to main placenta by fetal blood vessels within membrane
Prevalence: 0.14–3.00%
Cause: placental villi atrophy in area of inadequate blood supply → proliferate in two opposite directions (trophotropism) with fetal vessels remaining at the site of villous atrophy
| Cx: | (1) | Retention of accessory lobe in utero → postpartum hemorrhage |
| (2) | Placenta previa (= implantation over cervical os) → intrapartum hemorrhage | |
| (3) | Vasa previa (= connecting succenturiate vessels traversing internal os) may rupture → fetal blood loss |
2. Bilobed placenta
= 2 placentas of relative same size connected by a thin bridge of placental tissue
Risk: none
B. Placenta extrachorialis
= chorionic plate smaller than basal plate; ie, the transition of membranous to villous chorion occurs at a distance from the placental edge that is smaller than the basal plate radius
1. Circummarginate placenta
Frequency: up to 20% of placentas
Risk: no clinical significance
√ fetal membranes form a flat ring at site of attachment to chorionic plate
√ placental margin not deformed
2. Circumvallate placenta
= attachment of fetal membranes form a folded thickened ring with underlying fibrin + often hemorrhage
Prevalence: 1–2% of pregnancies
Risk: premature labor, threatened abortion, increased perinatal mortality, abruption, marginal hemorrhage
C. Placenta membranacea
= thin membranous placenta circumferentially occupying the entire periphery of chorion + presence of well-vascularized placental villi in the peripheral membranes ← failure of regression
Cause: ? endometritis, endometrial hyperplasia, extensive vascularization of decidua capsularis, previous endometrial damage by curettage
Risk: repeated vaginal bleeding extending into 2nd trimester; abortion at 20–30 weeks; postpartum hemorrhage
√ thickened outline over whole gestational sac (0.2–3.0 cm)
√ may show additional distinct disk of placenta
Placental Grading
= grading according to echo appearance of basal zone, chorionic plate, placental substance

◊ Premature placental calcifications are associated with cigarette smoking, hypertension, IUGR!
◊ Not considered useful because placental grading is imprecise for fetal dating or for fetal lung maturity!
GRADE 0
√ homogeneous placenta + straight line of chorionic plate
Time: < 30 weeks MA
GRADE 1
√ undulated chorionic plate + scattered bright placental echoes
Time: seen at any time during pregnancy; in 40% at term
◊ in 68% L/S (lecithin-sphingomyelin) ratio > 2.0
GRADE 2
√ linear bright echoes parallel to basal plate
√ confluent stippled echoes within placenta ± indentations of chorionic plate
Time: rarely seen in gestations < 32 weeks MA and in 40% at term
◊ in 87% L/S ratio > 2.0
GRADE 3
√ calcified intercotyledonary septa, often surrounding sonolucent center
Time: rarely seen in gestations < 34 weeks MA;
in 15–20% at term
◊ in 100% L/S ratio > 2.0 (= strongly correlated with lung maturity)
Premature Placental Senescence
= grade 3 placenta seen in gestation < 34 weeks MA
◊ In 50% suggestive of maternal hypertension / IUGR
Uteroplacental Circulation
By 20 weeks MA trophoblast invades maternal vessels and transforms spiral arteries into distended tortuous vessels = uteroplacental arteries
Physiology: maintains fetal pulmonary, hepatic, renal function by exchange of gas, metabolites, nutrients and indirect vascular interaction of fetal with maternal blood
Histo:
(a) in decidual segments of spiral arteries: proliferating trophoblast from anchoring villi invades lumen of spiral arteries + partially replaces endothelium
(b) in myometrial segments of spiral arteries: disintegration of smooth muscle elements → loss of elastic lamina → easily distensible vascular system of low resistance
Uterine Blood Volume Flow
› 50 mL/min shortly after conception
› 500–900 mL/min by term
Intervillous blood flow: 140 ± 53 mL/min (by 133Xe washout)
Umbilical Artery Doppler
Variables affecting Doppler measurements:
site of Doppler (close to placenta preferred), fetal heart rate, fetal breathing, drugs (eg, ritodrine hydrochloride decreases S/D ratio)
√ degree of diastolic flow increases as gestation progresses
› high-resistance flow @ < 20 weeks
› S/D ratio between 3.3 and 4.3 @ ≥ 20 weeks
› S/D ratio between 1.7 and 2.4 @ term
√ highly turbulent flow
IUGR Lesions
= narrowing of vascular lumen through
(a) thrombosis of decidual segments of uteroplacental aa.
(b) failure of development of myometrial segments of uteroplacental arteries
FETAL MENSURATION
◊ US is more reliable than LMP / physical examination!
Ultrasound Milestones
√ gestational sac w/o embryo or yolk sac = 5.0 weeks
√ gestational sac + yolk sac w/o embryo = 5.5 weeks
√ heartbeat ± embryo < 5 mm = 6.0 weeks
Accuracy: ± 0.5 week
Fetal Age
= GESTATIONAL AGE (GA) = “MENSTRUAL AGE” (MA)
= age of pregnancy based on woman’s regular last menstrual period (LMP) projecting the estimated date of confinement (EDC) at 40 weeks
◊ Note the inaccurate clinical usage of “gestational age,” which strictly speaking refers to the true (histologic) age of the pregnancy counting from the day of conception, whereas “menstrual age” refers to the (clinical) age of the pregnancy accounting for the ~ +2 weeks discrepancy!
◊ On subsequent US scans GA = GA assigned at 1st ultrasound + number of intervening weeks!
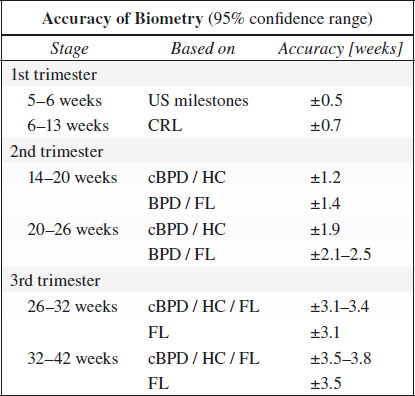
ACCURACY OF CLINICAL ASSESSMENT:
menstrual history ±2–3 weeks
1st-trimester exam ±2 weeks
fundal height ±4 weeks
Early Embryonic Size
= length of embryo < 25 mm on transvaginal scan performed at < 11 weeks MA
Gestational age (days) = embryonic size (mm) + 42
Accuracy: ± 3 days
Crown-Rump Length (CRL)
= length of fetus; useful up to 12 weeks MA (usually identified by 7 weeks MA on transabdominal scan)
Rule of thumb: MA (in weeks) = CRL (in cm) + 6
Accuracy: ± 5–7 days
Biparietal Diameter (BPD)
= measured from leading edge to leading edge of calvarial table at widest transaxial plane of skull = level of thalami + cavum septi pellucidi + sylvian fissures with middle cerebral arteries
◊ Excellent means of estimating GA in 2nd trimester > 12 weeks MA
Accuracy: 2 mm for “between occasion error”
◊ Most accurate for dating if combined with HC, AC, FL provided body ratios are normal!
◊ Less reliable for dating in 3rd trimester because of increasing biologic variability!
Discordant Estimated Date of Confinement (EDC) by LMP and BPD
1. Methodological error in measurement
(a) wrong axial section
(b) cranial compression (multiple gestation, breech presentation, oligohydramnios, dolichocephaly)
2. Erroneous LMP
other measurements (AC, FL) correlate with BPD
3. Abnormal head growth
(a) BPD < AC: microcephaly, fetal macrosomia
(b) BPD > AC: intracranial abnormality, asymmetric IUGR
Cephalic Index (CI)
= BPD / OFD; measurements of BPD and occipitofrontal diameter (OFD) both from outer to outer edge of calvarium
◊ Confirms appropriate use of BPD if ratio is between 0.70 and 0.86 (2 SD)
Corrected BPD (cBPD)
= BPD and OFD are used to adjust for variations in head shape
cBPD = √ BPD x OFD ÷ 1.26
Head Circumference (HC)
Used if ratio of BPD/OFD outside 0.70–0.86
| HC | = ([BPD + OFD]/2) x π |
| = ([BPD + OFD] x 1.62) x 3.1417 |
Accuracy: slightly less than for BPD
HC too large: hydrocephalus, hydranencephalus, intracranial hemorrhage, short limb dystrophies, tumor
HC too small: anencephaly, cerebral infarction, synostosis, microcephaly vera
Abdominal Circumference (AC)
= measured at level of vascular junction of umbilical vein with left portal vein (“hockey-stick” appearance) where it is equidistant from the lateral walls in a plane perpendicular to long axis of fetus; measured from outer edge to outer edge of soft tissues
◊ Allows evaluation of head-to-body disproportion
◊ Better predictor of fetal weight than BPD
AC too large: GI tract obstructions, obstructive uropathy, ascites, hepatosplenomegaly, congenital nephrosis, abdominal tumor
AC too small: diaphragmatic hernia, omphalocele, gastroschisis, renal agenesis
Femur Length (FL)
= measurement of ossified femoral diaphysis
Error: “flare” at distal end included in measurement (= reflection from cartilaginous condyle)
Thoracic Circumference (TC)
= measured in axial plane of chest, which includes four-chamber view of heart without inclusion of SQ tissue
◊ Linear growth between 16 and 40 weeks similar to AC
Useful age-independent parameter: TC÷AC > 0.80
Estimated Fetal Weight (EFW)
based on measurements of head size (BPD / HC), abdominal size (AD / AC), and femur length (FL)
Accuracy:

Appearance of Epiphyseal Bone Centers
in 95% of all cases
› distal femoral epiphysis (DFE): > 33 weeks GA
› distal femoral epiphysis (DFE) > 5 mm: > 35 weeks
› proximal tibial epiphysis (PTE): > 35 weeks GA
› proximal humeral epiphysis (PHE): > 38 weeks GA
CNS Ventricles
width of 3rd ventricle: < 3.5 mm (any gestational age)
Diameter of Cisterna Magna
measured from inner margin of occiput to vermis cerebelli: 2–10 mm
ASSESSMENT OF FETAL WELL-BEING
Amniotic Fluid Index
= sum of vertical depths of largest clear amniotic fluid pockets in the 4 uterine quadrants measured in mm
Method: patient supine, uterus viewed as 4 equal quadrants, transducer perpendicular to plane of floor + aligned longitudinally with patient’s spine
Variation: 3.1% intraobserver, 6.7% interobserver
Result:
› 95th percentile: 185 mm at 16 weeks GA, rising to 280 mm at 35 weeks, declining to 190 mm at 42 weeks
› 5th percentile: 80 mm at 16 weeks GA, rising to 100 mm at 23 weeks, declining to 70 mm at 42 weeks
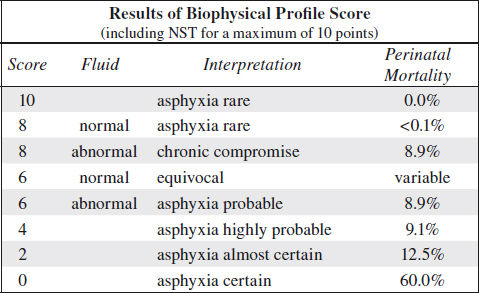
Biophysical Profile (Platt and Manning) = BPP
= in utero Apgar score = assessment of fetal well-being
Gestational age at entry: 25 weeks MA
Observation period: 30 (occasionally 60) min; ordinarily < 8 min needed; in 2% full 30 min required
A. ACUTE BIOPHYSICAL VARIABLES
◊ Subject to rhythmic variation coincident with sleep-wake cycle!
1. Fetal breathing movement (FBM):
√ ≥ 1 episode of chest + abdominal wall movement for at least 30 seconds (time is arbitrary to avoid confusion with general body movements / maternal respiration)
stimulated by: glucose, catecholamine, caffeine, prostaglandin synthetase inhibitor
suppressed by: barbiturates, benzodiazepine, labor, hypoxia, asphyxia, prostaglandin E2
2. Fetal body movement:
√ ≥ 3 discrete movements of limbs / trunk
Influenced by: glucose, gestational age, time of day, maternal drugs, intrinsic rhythm, labor
3. Fetal tone
upper + lower limbs usually fully flexed with head on chest; least sensitive test parameter
√ ≥ 1 episode of opening + closing of hand / extension + flexion of limb
B. CHRONIC FETAL CONDITION
4. Amniotic fluid volume
√ at least one pocket ≥ 2 cm in vertical diameter in two perpendicular planes
◊ Avoid inclusion of loops of cord!
BPP Score
for each test: 2 points if normal; 0 points if abnormal
False-negative rate: 0.7÷1,000
◊ The probability of fetal death within a week of a BPP score of 8/8 is 1÷1,000!
Stress Tests
Nonstress Test (NST)
◊ Test needed in less than 5% of cases!
√ reactive fetal heart rate tracing (normal) = at least 4 fetal heart accelerations (> 15 bpm over baseline lasting > 15 seconds) in a 20-minute period subsequent to fetal movement > 34 weeks GA
√ nonreactive (abnormal) fetal heart rate tracing = absence of acceleration in a continuous 40-min observation period
N.B.: no heart accelerations in immaturity, during sleep cycle, with maternal sedative use
Accuracy: false-negative rate of 3.2÷1,000 (if done weekly) or 1.6÷1,000 (if done biweekly); 50% false-positive rate for neonatal morbidity + 80% for neonatal mortality
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree