Description and Causes
Malformations involving the vein of Galen are rare congenital connections occurring between intracranial arteries (usually thalamoperforator, choroidal, and anterior cerebral arteries) and the vein of Galen or other primitive midline vein (
37,
69). These connections can be large direct fistulas, numerous small connections, or a combination thereof. The cause of these connections is unknown. Some investigators have noted a strong association with venous anomalies (absent straight sinus, persistent falcine and occipital sinuses) and suggested that intrauterine straight sinus thrombosis with recanalization is responsible (
69). Raybaud et al. demonstrated that the dilated primitive venous structure represents persistence of the embryonic median prosencephalic vein of Markowski (
89). They suggested that early obstruction of the straight sinus might result in persistence of the primitive veins based upon the need for venous drainage pathways. Moreover, in our experience, vein of Galen malformations are associated with certain cardiovascular anomalies, most commonly aortic coarctation and secundum atrial septal defects (
90).
Over 90% of the vein of Galen varices falls into the group called “choroidal” malformations (
91). Choroidal malformations are arteriovenous connections from a plethora of vessels, usually numerous choroidal, pericallosal, and thalamoperforator vessels, to the anterior wall of the prosencephalic vein, resulting in a great deal of arteriovenous shunting (
92,
93); the consequence of the shunting is presentation as neonates with CHF. Choroidal malformations have the poorest prognosis, and are usually fatal without treatment. The second, less common, category of vein of Galen malformation is the so-called “mural” malformation (
91). Mural malformations are characterized by fewer (usually one to four) but larger caliber connections with the prosencephalic vein; the posterior choroidal or collicular arteries are most commonly involved. Patients with mural malformations usually present in infancy with developmental delay, hydrocephalus, and seizures but mild or no signs of CHF. The treatment approach to these malformations varies although endovascular therapy has become the method of choice for both and offers a high rate of cure with low morbidity (
94,
95,
96).
Clinical Presentation and Imaging Findings
The clinical presentation of vein of Galen malformations can be categorized into three groups: (i) the neonate presenting with intractable CHF and loud intracranial bruit, (ii) the infant presenting with hydrocephalus and/or seizures, and (iii) the older child or young adult presenting with hemorrhage (
97). As mentioned in the preceding section, patients in group 1 typically have choroidal malformations, whereas patients in groups 2 and 3 have mural malformations.
With improvements in quality and availability of prenatal imaging, many vein of Galen malformations are now diagnosed antenatally (
98,
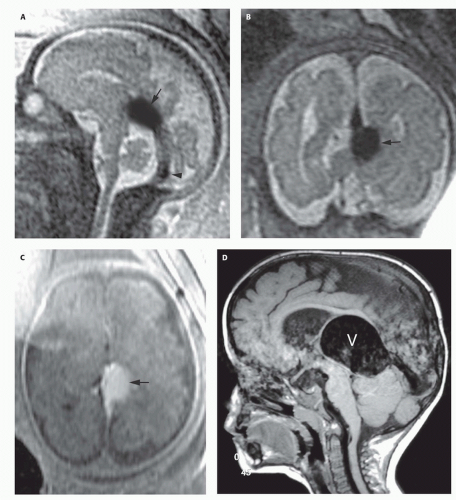
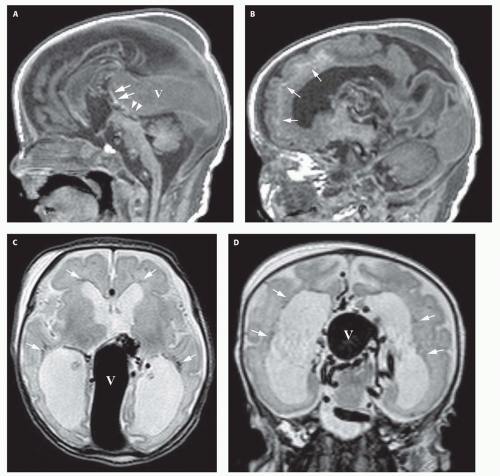
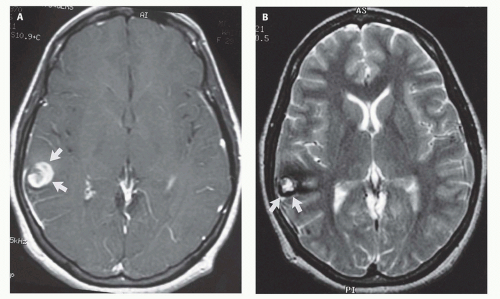
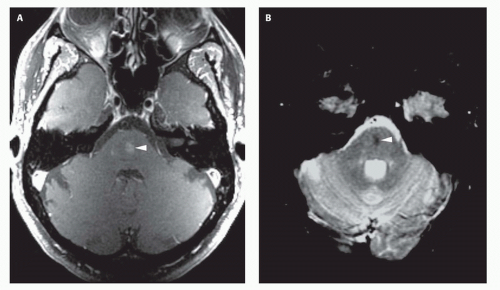
99). Prenatal sonograms show a large hypoechogenic to mildly echogenic midline mass that is seen to have rapid flow on Doppler studies. MRI identifies the varix best (
Fig. 12-4) and feeding vessels, and can be used to identify any associated structural abnormalities such as malformation or ischemic injury. If such a patient is identified, the interventional neuroradiology service must be alerted and available to provide assistance at the time of delivery and in the perinatal period. If the patient is found to have neonatal heart failure that is refractory to medical therapy, the neurointerventionalist may need to treat these patients (
100).
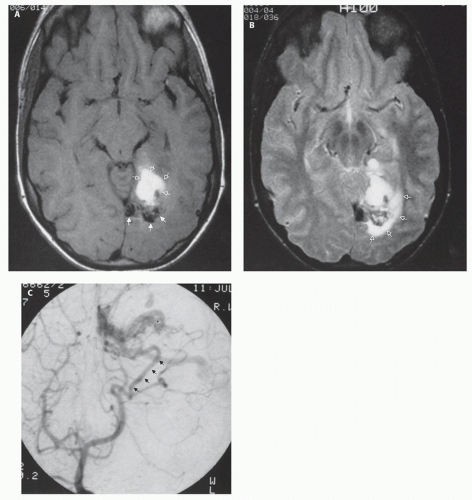
If the vein of Galen malformation is not diagnosed prenatally, postnatal neuroimaging becomes critical to making the proper diagnosis. On imaging studies, the dominant feature of the vein of Galen malformation is the varix, which appears as a large mass in the incisural region, sometimes extending rostrally and anteriorly displacing the third ventricle (
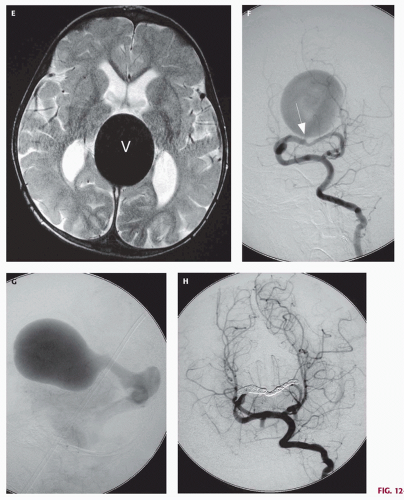
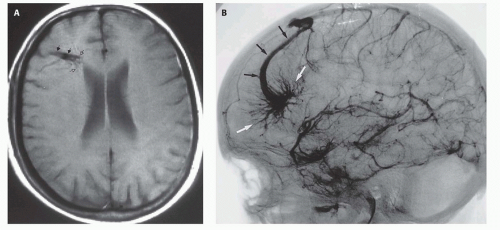
Fig. 12-4). On sonography, the varix will appear mildly echogenic on two-dimensional (2D) imaging and show turbulent flow on Doppler (
Fig. 12-5); it is important to demonstrate continuity with the straight sinus or a persistent falcine sinus. Doppler studies may be useful to quantify the rate of flow within the varix for future reference. On CT, the varix will be iso- to hyperdense to brain prior to contrast administration (
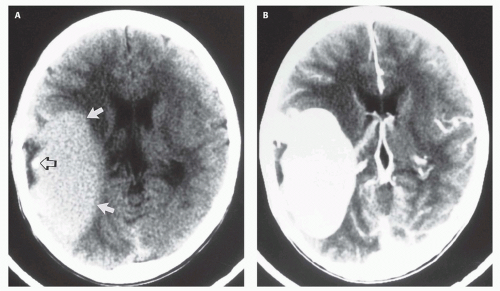
Fig. 12-5). Mixed attenuation may be seen if the varix is partially thrombosed. Areas of low attenuation (encephalomalacia, usually secondary to ischemia) and high attenuation (hemorrhage or dystrophic calcification) are often present in the brain parenchyma. The extent of brain injury should be carefully analyzed, and the parents informed of likely neurological and developmental sequelae, before therapy is begun. On MRI, the varix will be hypointense (
Fig. 12-4) resulting from a loss of phase coherence of the mobile protons; mismapped signal will often be seen across the image in the plane of the phase-encoding gradient (
Figs. 12-4 and
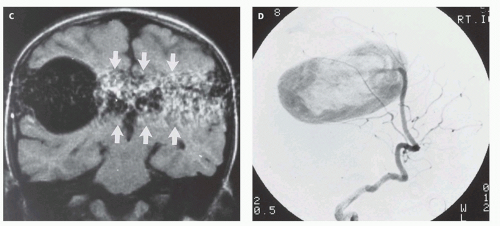
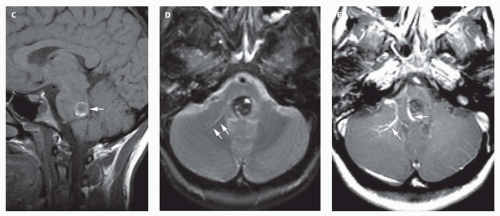
12-6). Feeding vessels will be identified on axial images as round areas of signal void in the ambient cisterns and on MRA as thin, curvilinear structures demonstrating flow-related enhancement and connecting to the varix. Areas of acute thrombosis will usually be isointense to brain on T1-weighted sequences and hypointense on T2-weighted sequences, whereas subacute thrombus will have a high intensity on both T1- and T2-weighted sequences. Thrombus of varying age usually lines the wall of the varix. Areas of damaged brain will typically appear hyperintense on T1-weighted images and hypointense on T2-weighted images in the neonate (
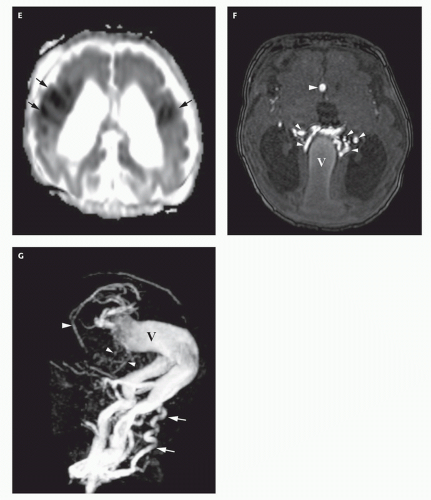
Fig. 12-6); they may be difficult to see on FLAIR. Diffusionweighted images will show acute injury as regions of reduced diffusivity (
Fig. 12-6). As the brain begins to myelinate, injured brain is better seen on T2-weighted images and then on FLAIR images (see explanations in
Chapter 4). If left untreated, the fistula will continue to grow, recruiting additional blood supply and engendering new fistulas (
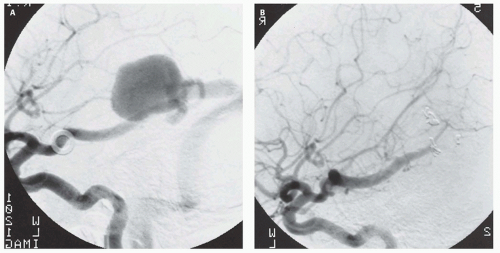
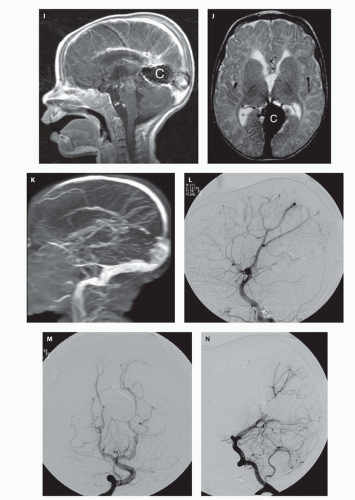
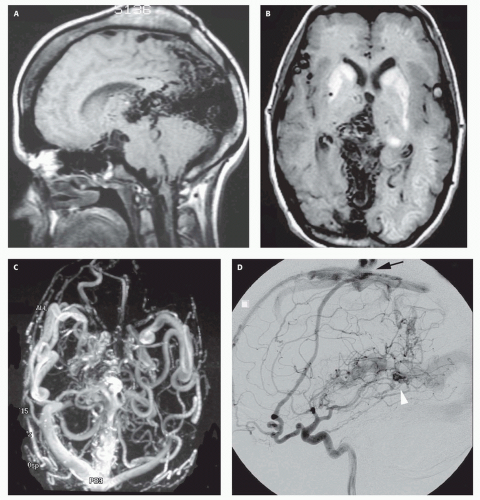
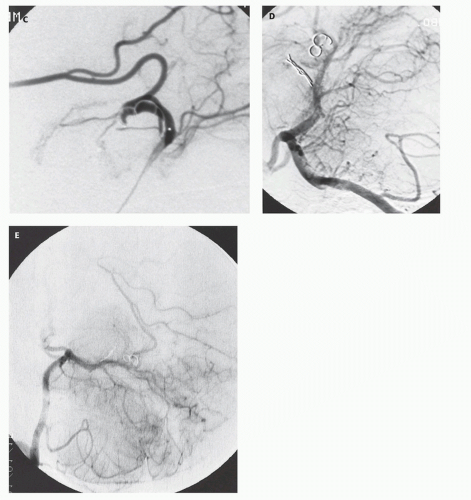
Fig. 12-7).
Treatment
Aggressive medical management of the cardiac failure associated with Galenic malformations is an essential adjunct to surgery or endovascular procedures, but medical management alone can rarely control the failure. Johnston’s review of neonates presenting with CHF revealed a mortality of 95%, and none were stabilized without surgical intervention (
101). Surgical ligation of the anomalous connections has been described, but the results have been disappointing. In a review of 60 neonates treated by surgery, there were only six survivors; half of the survivors suffered from neurological deficits (
101). Several series have reported the efficacy of endovascular procedures as a palliative or definitive treatment (
102,
103,
104). In the early 1980s, these procedures consisted of free particle embolization. While the majority of these emboli would lodge in the fistulous connections, the risk of an errant embolus occluding a normal cerebral blood vessel was inversely proportional to the flow in the fistula, and the majority of these procedures were palliative. With the development of newer microcatheter delivery systems and embolic agents such as platinum coils (
105), silk sutures, and liquid adhesives (
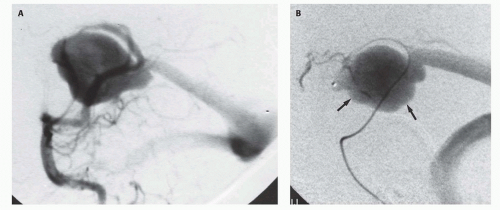
91), superselective embolization of the fistula connections alone can be achieved (
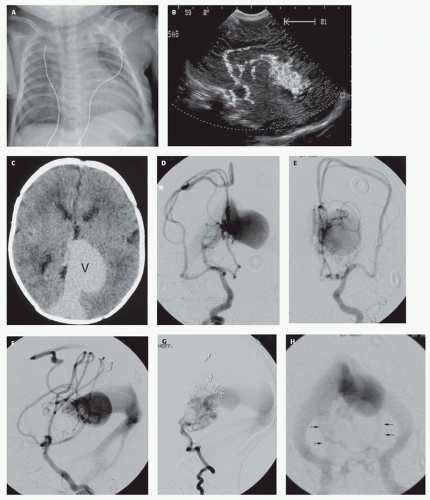
Figs. 12-5 and
12-8). Mickle et al. developed a technique where the torcular is surgically exposed, a small catheter is placed transvenously through the straight or falcine sinus into the involved vein of Galen, so metal coils can be deposited to diminish the arteriovenous shunting (
104). Although useful in the presence of bilateral transversesigmoid or occipital sinus hypoplasia or occlusion, the transtorcular approach is not often necessary, as embolic agents can be placed via transfemoral venous access.
Over the past 10 years, 34 children with Vein of Galen malformations have been treated at our institution; 26 harbored the choroidal variety and presented with CHF (
94,
103,
106,
107). The first five patients were treated by craniotomy and attempted clipping of the feeding
vessels. All five patients died during or shortly after the surgery. The subsequent eight patients underwent transvascular embolization techniques. Six of the eight survived while two died despite treatment (
103). Of the survivors, one suffered a severe middle cerebral infarct resulting from an errant embolus. Another had a partial visual field deficit, presumably a result of ischemic damage secondary to the underlying disease. The remaining patients are neurologically and developmentally normal with marked reduction of the fistula flow following treatment. At long-term independent follow-up, 61% of patients who survived their initial presentation were neurologically normal or demonstrated only minor developmental delay (
96).
Improvements in technique and embolic materials allow vein of Galen malformations to be treated by transarterial or transvenous approaches. These new materials and approaches have resulted in a high rate of successful therapy; indeed, greater than 50% angiographic cure and 75% symptomatic improvement have been achieved in our recent patients, even in those with the high-flow choroidal malformations (
106,
107). Our cure rate is 100% for the less common mural type of vein of Galen malformation that most commonly presents later in infancy with hydrocephalus, seizures, and failure to thrive (
94).
The following are the current recommendations for treatment of vein of Galen malformations presenting with severe congestive failure.
If the diagnosis is established prenatally, the delivery should be performed at an institution offering endovascular techniques to palliate the patient should intractable CHF develop. Severe heart failure
in utero can result in polyhydramnios and hydrops fetalis, which can be an indication for induced delivery. Close coordination among the obstetricians, neurointerventionalists, neonatologists, and neurosurgeons is essential to optimize planning. Baseline ultrasonography with color flow Doppler should be performed to serve as a baseline for blood flow in evaluating the results of the endovascular techniques. If possible, umbilical arterial and venous catheters should be placed at the time of delivery to allow repeated vascular access for both diagnostic and therapeutic procedures. These indwelling catheters obviate the necessity for repeated femoral punctures in the fragile neonatal femoral artery. A CT or MRI should be performed to assess any parenchymal damage already produced by the congenital fistula, disclose hydrocephalus, which may require ventriculoperitoneal shunting, and serve as a baseline.
If intractable congestive failure persists despite aggressive medical management, angiography is performed to delineate the vascular anatomy. Palliative arterial embolization can be performed at this time, preferably with superselective catheterization of each feeding pedicle to reduce the risk of ischemic damage to normal surrounding brain. The embolization procedure may be repeated if congestive failure persists. If the congestive failure continues and further arterial embolization is considered risky or technically impossible, transvenous embolization may become necessary. An arteriogram is performed to localize the draining venous sinuses. If the draining venous sinuses are patent, the transfemoral or transumbilical routes can be used to access the varix. On rare occasions, when the lateral sinuses are absent or severely hypoplastic, surgical access to the intracranial venous system is necessary. A small burr hole is made over the draining falcine or straight sinus. A needle puncture is made into the sinus, a catheter advanced into the varix, and platinum coils deposited. These techniques can be curative or palliative. When palliative, they alleviate the congestive failure and allow the child to develop normally until a definitive treatment can be performed with further surgical or radiological techniques. MRI scanning is useful in our experience to assess brain development, the degree of thrombosis in the fistula site, and delayed development of hydrocephalus.