Neoplasms of the Spine
A. James Barkovich
Tumors of the spinal cord in children are slowly growing masses with clinical manifestations that are often subtle and slowly progressive. The widespread use of magnetic resonance imaging has greatly facilitated the diagnosis of spinal tumors, allowing earlier detection and therapy. Although the neoplasms that involve the pediatric spinal cord are similar to those affecting adults, the incidence and the presentation of the various tumors are often quite different. In this chapter, the epidemiology, clinical manifestations, and pathologic/radiologic manifestations of intraspinal neoplasms in children are discussed.
 General Imaging Characteristics of Spinal Tumors
General Imaging Characteristics of Spinal TumorsThe introductory section of Chapter 7 discussed general characteristics of brain tumors. In that discussion, the importance of determining whether a tumor is located within the brain (intraparenchymal) or outside of the brain (extraparenchymal) was stressed. A similar distinction must be made in tumors of the spine: does the tumor originate within the spinal cord (intramedullary tumor) or outside of the spinal cord (extramedullary tumor)?
Differentiating an intramedullary mass from an extramedullary mass by imaging is usually straightforward. Intramedullary masses cause enlargement of the spinal cord as viewed in the sagittal, coronal, and axial planes. The spinal cord above and below the mass remains in its normal location within the spinal canal. The margins of the intramedullary mass may be sharply defined (particularly in congenital tumors, such as dermoids and epidermoids) or indistinct (particularly in infiltrating astrocytomas). Extramedullary masses always appear sharply marginated in at least one imaging plane. When small, they are usually clearly separated from the cord by CSF. When large, the CSF space between the mass and the cord is obliterated. In addition, the mass may compress the cord. If compressed by a laterally located mass, the cord will appear enlarged if viewed in the sagittal plane; therefore, coronal and axial planes are necessary to make the distinction. If compressed by an anteriorly or posteriorly located mass, the cord will appear enlarged in the coronal plane; imaging in the sagittal and axial planes will allow diagnosis. Sometimes, the site of tumor origin is clarified after tumor enhancement by intravenous administration of contrast.
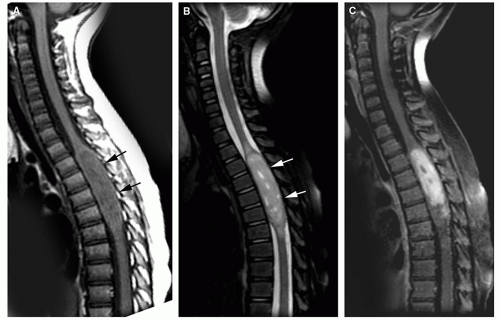
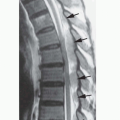
A potential pitfall in the diagnosis of intramedullary tumors is in the differentiation of tumors from nonneoplastic processes in the spinal cord. In children, the major problem is in the differentiation of tumors from foci of demyelination. For example, children with acute disseminated encephalomyelitis or multiple sclerosis (see Chapter 3) can present with signs and symptoms referable to a spinal cord lesion. In either disease, a region of focal cord enlargement with prolongation of T2 relaxation time may be identified. Inflammatory lesions (e.g., demyelinating plaques) may disturb the blood-brain barrier and enhance after the administration of contrast. Perfusion imaging, which helps in this differentiation in brain tumors (see Chapter 5), is not yet practical in the spine because of susceptibility effects from the spinal column and the small size of the spinal canal. Therefore, inflammation is most easily differentiated from tumor by assessing the shape and size of the lesion at the time of presentation. Neoplasms of the spine are generally round to ovoid in shape (sometimes with irregular borders) and often have associated cysts (Fig. 10-1A and B). They typically cause considerable enlargement in the cord diameter at the time of presentation because they rarely cause symptoms when they are less than 2 cm in diameter. Inflammatory lesions vary somewhat with the cause (parainfectious inflammatory lesions are often very elongated, while plaques of demyelination from multiple sclerosis are usually flame shaped), but they rarely cause significant enlargement of the spinal cord (Fig. 10-1C and D). Associated cysts are almost never present. It may be helpful to image the brain to look for other characteristic foci of demyelination (see Chapter 3); if present, they establish the diagnosis. If no other lesions are found, and uncertainty persists as to whether an intramedullary lesion is tumor or plaque, a follow-up MR exam with and without paramagnetic contrast should be obtained 4 to 6 weeks after the initial exam. Tumor will be unchanged or larger, whereas a focus of demyelination should be smaller and the enhancement characteristics should be different than on the first study. A delay of 6 weeks will not impact on the outcome of a patient with a tumor, but biopsy of an acute inflammatory lesion may leave the patient with a worse neurological deficit.
 Intramedullary Tumors
Intramedullary TumorsClinical Presentation
Intramedullary (intrinsic) neoplasms of the spinal cord constitute 4% to 10% of neoplasms of the central nervous system in children (1,2,3,4) and approximately one-third of spinal neoplasms of childhood (2,5,6,7). All age groups are affected; however, intramedullary tumors are most frequently seen in children toward the end of the first decade
and beginning of the second decade of life. Males and females are equally affected (8,9,10,11). Spinal neoplasms are relatively less common in children than adults; the ratio of intracranial to intraspinal neoplasms in children varies between 10:1 and 20:1 (as compared to 5:1 in adults) (11).
and beginning of the second decade of life. Males and females are equally affected (8,9,10,11). Spinal neoplasms are relatively less common in children than adults; the ratio of intracranial to intraspinal neoplasms in children varies between 10:1 and 20:1 (as compared to 5:1 in adults) (11).
The availability of MR has facilitated the diagnosis of spinal tumors; nonetheless, the patient’s course often will have been punctuated by exacerbations and remissions of symptoms, possibly due to alteration in the edema surrounding the neoplasm (6). Weakness with numbness or pain is the most common presenting complaint (11,12). The most common type of pain, occurring in approximately 70% of patients, is spinal pain. This pain is dull and aching in quality and is localized to bone segments adjacent to the tumor (11). The cause of this pain is unknown but may result from distention of the dural tube by the swollen spinal cord (11). Sometimes children will complain of nocturnal pain that awakens them from sleep (6). The second most common type of pain seen in children is radicular (root) pain, which is indistinguishable from pain caused by compression of a nerve root by an extramedullary process (11). Finally, some patients manifest tract pain, a vague, burning type of pain associated with paresthesias. The burning and paresthesias typically occur caudal to the tumor and are believed to result from infiltration of the lateral spinothalamic tracts by tumor (11).
Children of different age groups often present with very different symptomatology. Young children and infants often have severe pain, motor regression, weakness, or frequent falls (2,6), whereas older children more commonly present with clumsiness, progressive scoliosis (thoracic tumors), torticollis (cervical tumors), gait disturbance, sphincter disturbance, or spinal deformity (4,11,12,13). Weakness of the extremities is a common finding in all patients. In tumors of the cervical spinal cord, the most common location in children, upper extremity weakness may or may not be accompanied by weakness of the lower extremities, neck pain, muscle atrophy, dysesthesias, or hyperreflexia (4). Although sensory levels may be present, they are less common in intramedullary than extramedullary tumors. Sphincter dysfunction, when present, may not occur until late in the course of the illness (9,14,15); early presentation may indicate a high-grade (fast-growing) tumor (16).
Up to 15% of patients with spinal cord tumors may present with symptoms of increased intracranial pressure, most likely a result of either greatly elevated CSF protein content, which blocks the normal CSF pathways and impairs CSF resorption, or blockage of the foramen magnum by cervicomedullary tumors (see Chapter 8) (12,17,18). The increased intracranial pressure does not misdirect the diagnostic workup if the patients have signs of an intraspinal process on physical exam. However, patients having no signs or symptoms of a spinal process may suffer from a considerable delay in diagnosis.
Pathology
The most common histologic type of spinal cord tumor in children is astrocytoma (60%), followed by ependymoma (15%-30%) (3,6,12,19,20,21,22). Thus, the relative incidence of intramedullary spinal cord tumors in children is quite different from that in adults, in whom more than half of intramedullary tumors are ependymomas. Another difference from adults is that the myxopapillary ependymoma, a benign (WHO Grade I) form of ependymoma that constitutes 40% to 50% of spinal ependymomas in adults (usually at or below the conus medullaris), is rare in children, composing only 8% to 12% of spinal ependymomas in childhood (21,23,24); most pediatric ependymomas appear to be WHO Grade II (cellular, papillary, epithelial, or clear cell). Malignant astrocytomas and glioblastomas of the cord are relatively more common in children than in adults, constituting as many as 50% of pediatric astroglial spinal cord tumors (25,26). Other intramedullary tumors may develop in children, including gangliogliomas and gangliocytomas (27,28,29), germinomas (3,19,30,31), primitive neuroectodermal tumors (PNETs) (32,33), atypical teratoid/rhabdoid tumors (16,34,35,36), oligodendrogliomas (37), pleomorphic xanthoastrocytomas (19,38), melanocytomas (19), teratomas (39), and Langerhans cell histiocytosis (3,40). Very rarely, intramedullary tumors may be metastases from fourth ventricular tumors (that presumably seed via the obex into the central canal of the spinal cord) (41).
Just as with their intracranial counterparts, the extent of resection of spinal tumors is the primary determinant of the rate of recurrence and the expected length of survival (42). Astrocytomas are generally more difficult to completely resect than are ependymomas; thus, patients with astrocytomas have poorer postoperative courses and shorter survival (12,43,44). Histology of the tumor is also an important factor in determining expected survival. In one study, 10-year survival for pilocytic astrocytomas of the spinal cord was 81% as compared to 15% for diffuse fibrillary astrocytomas (45) and even worse for glioblastoma multiforme, where subarachnoid spread is found in up to 60% of cases (46). Among patients with fibrillary astrocytomas, the grade of the tumor was the most significant predictor of survival (45). Among ependymomas, too, high tumor grade (but not histology—there does not appear to be any difference in the biologic behavior of the different histologic types of WHO Grade II ependymomas (3)) was the most significant variable for prediction of dissemination through the cerebrospinal axis (47); dissemination is a grave prognostic factor.
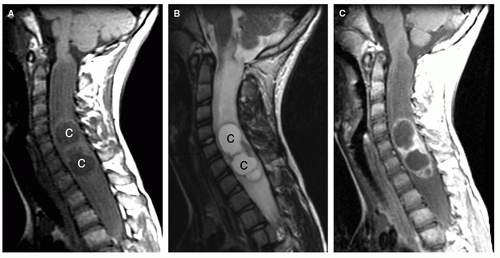
Intramedullary neoplasms tend to be located more rostrally in children than in adults (1,11). Nearly 50% of intramedullary tumors in children are cervical or cervicothoracic, as compared with 28% in adults. Intramedullary tumors in children usually occupy a small number of cord segments; however, the extent of cord involvement is often increased by the presence of cysts rostral or caudal to the tumor. Such peritumoral cysts have been reported in 40% of spinal cord astrocytomas (48) and in up to 80% of ependymomas (49,50).
Imaging
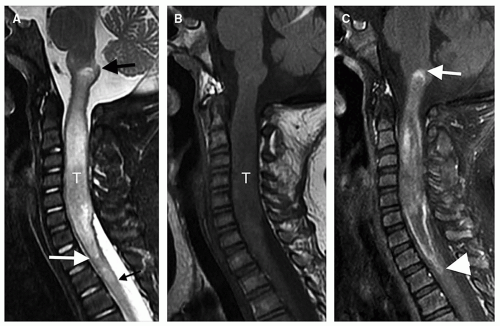
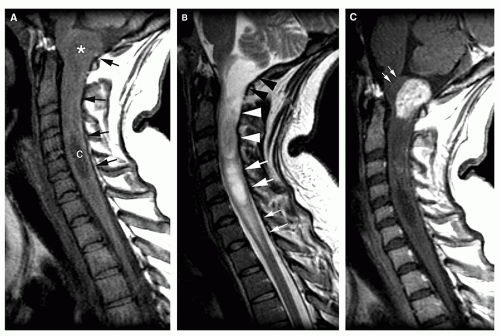
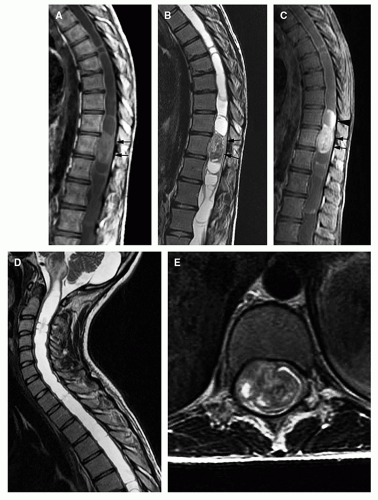
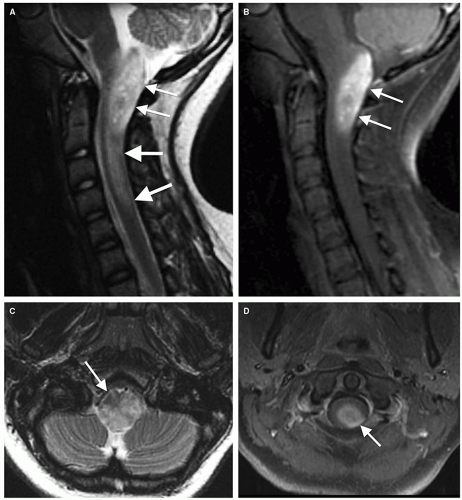
Magnetic resonance is the modality of choice for imaging patients with suspected or known intramedullary neoplasms. MR of most intramedullary tumors will show an enlarged spinal cord with T1 hypointensity and T2/FLAIR hyperintensity at the location of the tumor (Figs. 10-1, 10-2, 10-3, 10-4, 10-5, 10-6 and 10-7) (22,51,52). As in the brain, tumors with high cell density, such as PNETs and atypical teratoid/rhabdoid tumors, have relatively little free water and therefore show less T2 hyperintensity (32,36,53), although this characteristic seems less reliable in the spinal cord than in the brain (54). The regions of abnormal T1 hypo- and T2 hyperintensity within the expanded cord may represent tumor, necrosis, cyst, or edematous cord (“presyrinx,” Fig. 10-1) (55); therefore, intravenous administration of paramagnetic contrast (Figs. 10-1, 10-2, 10-5, 10-6 and 10-7) is essential. In 20% to 40% of patients, cysts are present caudal or rostral to the solid portion of the tumor (Figs. 10-1 and 10-5) (11,48,56); this number may be as high as 80% in ependymomas (49,57,58). These cysts may result from secretion of fluid by the tumor or by obstruction of normal CSF pathways within the cord and subarachnoid space; the latter should be considered syringomyelia (see Chapter 9). The solid portion of the tumor often cannot be differentiated from the cyst without intravenous administration of contrast (Fig. 10-5). Axial and sagittal T1-weighted sequences may be of use in defining the full extent of the associated cyst. The full extent of the cyst is not critical information, however, because it usually resolves after resection of the tumor and elimination of the obstruction. Contrast enhancement is usually nodular, heterogeneous (Figs. 10-2, 10-4, and 10-5), or ring-like (Fig. 10-7); although there are exceptions (59,60), enhancement is seen in the large majority of intramedullary spinal neoplasms in children (22,51,52,61). The region of contrast enhancement corresponds to the solid portion of the tumor and usually assists in distinguishing the solid portion of the tumor (which typically enhances) from cyst and necrosis (which do not enhance, although contrast may leak into a cyst lined by tumor as in Figure 10-5).
Astrocytomas (Figs. 10-1 and 10-2) are usually located more eccentrically in the spinal cord and enhance with variable intensity (sometimes, not at all), and the enhancement is less sharply demarcated from the nonenhancing tissue than in ependymomas (Figs. 10-4 and 10-5) (62). The tumor margins of astrocytomas usually extend beyond the enhancing tissue.
Ependymomas are usually located centrally within the spinal cord (Figs. 10-4 and 10-5); the margin of tumor enhancement is sharper and corresponds to the margin of solid tumor (23,24,58). A finding of T2 hypointensity at the superior and inferior borders of the mass (Fig. 10-5), indicating prior hemorrhage, is suggestive of ependymoma (63); this sign is seen in only about 20% of ependymomas, however (50). Remember that imaging findings of intramedullary tumors are not always diagnostic: in a large series, the presence of associated syringomyelia (rostral or caudal cyst) was the only imaging sign that independently differentiated ependymoma from astrocytoma (58), and it is not 100% diagnostic.
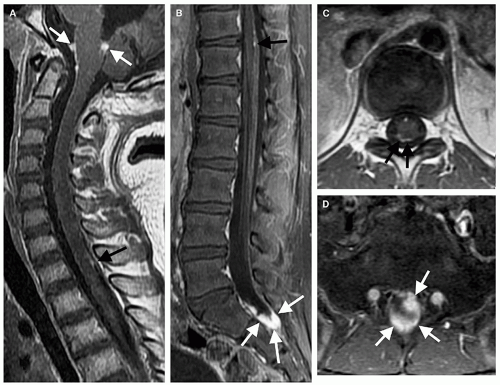
Germinomas and gangliogliomas (Figs. 10-6 and 10-7) are typically sharply circumscribed, as well. Heterogeneity of the tumor on T1-weighted images is said to be suggestive of ganglioglioma (Fig. 10-6) (49), although astrocytomas may have a similar appearance (Fig. 10-1). No specific MR characteristics allow differentiation of benign from malignant spinal cord tumors in children, although the presence of leptomeningeal spread at the time of presentation suggests malignancy (64). In practice, preoperative differentiation of these tumors is of little clinical importance because the operative approach does not differ. It is much more important to determine whether the tumor is intramedullary or extramedullary and to accurately identify the level of the solid portion of the tumor.
The differentiation of tumor-associated syringomyelia from congenital or posttraumatic syringomyelia is difficult, even impossible, without administering paramagnetic contrast. Edema with reactive astrogliosis, which has the same signal characteristics as tumor (T1 hypointensity and T2 hyperintensity), is frequently present at the caudal and rostral ends of benign syrinx cavities (see Chapter 9) (65,66); this finding may be a “presyrinx” that is slowly evolving into true syringohydromyelia (55). Although the presence of anomalies of the craniocervical junction such as cerebellar tonsillar ectopia, occipitalization of the atlas, and the Klippel-Feil anomaly suggest that a syrinx is nonneoplastic, these anomalies can occur in conjunction with spinal cord tumors as well (65,67). Moreover, the expansion of the intramedullary tumor with resulting obstructed CSF flow in the spinal subarachnoid space can cause a syringohydromyelia (Figs. 10-2 and 10-5) identical to that seen associated with tonsillar ectopia (65,66) or other causes of obstruction of the subarachnoid spaces (55). Patients with typical syringohydromyelia (see Chapter 9) and an obvious cause for the syrinx, such as a Chiari I malformation, do not need a postcontrast MR sequence. However, if any question exists regarding the cause of the syrinx, paramagnetic
contrast should be administered to look for enhancing tumor. If an area of contrast enhancement is present within or adjacent to the syrinx, the diagnosis of tumor-related cyst should be strongly considered. Rarely, an enhancing inflammatory mass can cause cavitary cord disease.
contrast should be administered to look for enhancing tumor. If an area of contrast enhancement is present within or adjacent to the syrinx, the diagnosis of tumor-related cyst should be strongly considered. Rarely, an enhancing inflammatory mass can cause cavitary cord disease.
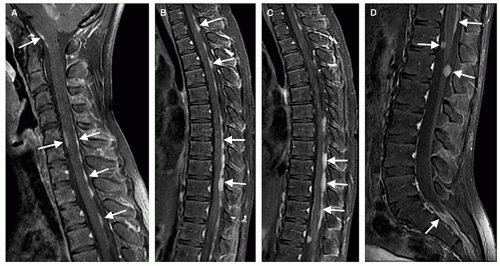
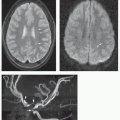
Rarely, intramedullary tumors have disseminated throughout the subarachnoid spaces by the time of presentation; the imaging appearance is identical to that of CSF spread from intracranial tumors (Figs. 10-8 and 10-9) (37,38,68). Although this finding is generally associated with higher tumor grade, dissemination may be found in lowgrade tumors, as well, particularly those that develop in CSF spaces (choroid plexus tumors, ependymomas, medulloblastomas, etc.) (37,68,69). Such patients may initially come to medical attention because of signs of increased intracranial pressure. It is important to remember, as discussed in Chapter 8, that CSF dissemination of spinal tumors can cause hydrocephalus and that the spine should be investigated in children (or adults) with unexplained hydrocephalus who worsen despite CSF diversion.
Congenital intramedullary tumors, such as lipomas, teratomas, hamartomas, dermoids, and epidermoids, are discussed in Chapter 9.
 Extramedullary Tumors
Extramedullary TumorsApproximately two-thirds of all intraspinal tumors of childhood are extramedullary; of these, 50% are extradural and 10% to 15% are intradural (70,71). Extramedullary neoplasms can be classified by a number of different criteria; they will be classified in this chapter by their site of origin. Clinically, patients with extramedullary tumors present with signs of progressive myelopathy. Presentation is similar to intramedullary tumors in that weakness, predominantly of the lower extremities and trunk, is the most common presenting symptom. Diffuse back pain and radicular (“root”) pain are frequently present. Torticollis, upper extremity weakness, scoliosis, and urinary incontinence are other common signs and symptoms (10,72). The histological type of tumor cannot be predicted by its clinical presentation.
CSF Dissemination of Intracranial Neoplasms
Any tumor that is in the cerebral ventricles or subarachnoid space, benign or malignant, can spread via the CSF. The dissemination of tumor from intracranial neoplasms via the CSF occurs more frequently in the pediatric age group than in adults. This manner of tumor spread is most commonly observed in medulloblastomas (73). Up to one-third of patients with medulloblastoma will eventually disseminate tumor through the CSF, most often to the spine (74); this spread via CSF appears to be more common in children with group 3 and group 4 subtypes (75,76). These groups and, therefore, the presence of metastases in the spinal canal indicate a much poorer prognosis for the patient (75).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree