Techniques for Pediatric Neuroimaging
Christopher P. Hess
Duan Xu
A. James Barkovich
The past two decades have seen tremendous advances in the field of pediatric neuroimaging. Improvements in technology, broader recognition of the risks of sedation and radiation, and enhanced access to high-quality imaging across the medical community have significantly altered referral patterns and imaging strategies for evaluating disorders of the brain, head, neck, spine, and peripheral nervous system. In addition, in the United States, overall shifts in the payment landscape for national health care have renewed focus on achieving best practices for neuroradiology, and the term “value” has entered the routine lexicon of academic and community practices for medical diagnostics. Although the same principles that have been used to guide the use of imaging for many years still apply, the future of pediatric neuroradiology must now be viewed more carefully through the lenses of quality, safety, and cost efficiency.
Radiologists have long appreciated the significant differences between imaging children and imaging adults. The pace and magnitude of developmental changes that occur in the brain and spine, nuanced by the different spectrum of neurologic disease in children, require tailoring of imaging protocols to optimally detect and show the anatomic, physiologic, and metabolic changes in pediatric disorders. Coils, scanning parameters, and approaches for sedation and monitoring differ significantly for imaging the fetus, the newborn, the infant, and the adolescent. Far from “one size fits all,” the choice of modality and specific imaging technique that should be applied depends upon the age and medical condition of the patient, the indication for imaging, the urgency of diagnosis, and the cost of imaging.
This chapter outlines techniques for safe, high-quality, and effective neuroimaging in pediatric patients. First, we outline special considerations for imaging, including sedation, selection of appropriate imaging modality, and the use of intravenous contrast agents. The use of cranial ultrasound and CT is then reviewed. Given the narrow window of situations in which these modalities are now applicable, these sections are brief. The remainder of the chapter focuses on MR, the current clinical standard for evaluation of the brain and spine. We consider first anatomic imaging and then pivot to physiologic and metabolic MR techniques. Specific approaches to pediatric disease that are used in our own practice at UCSF are outlined. The techniques that are discussed in this chapter will be referred to throughout the remainder of this book.
 Special Considerations for Imaging Children
Special Considerations for Imaging ChildrenNeonates
Premature infants present the special problems of small size and inability to maintain constant body temperature. In general, premature infants should be imaged initially with ultrasound in the neonatal intensive care unit. Cranial ultrasound is the initial examination of choice in these patients because it is inexpensive and portable (exams can be performed without moving infant from the neonatal intensive care unit). Moreover, transfontanelle ultrasonography with high-frequency transducers is excellent for the detection of edema, blood, or infarction in deep structures of the brain, the location of most central nervous system pathology in the premature infant, and for development or progression of hydrocephalus. However, MR has an increasing role in the evaluation of the premature infant, as it can detect abnormalities that are not visible by sonography (1,2) and these abnormalities are of prognostic significance (3,4,5,6).
When an MR examination is necessary, special precautions must be observed to ensure the safety of the neonate. It is best to enlist the assistance of neonatologists and/or neonatal nurses in the transport of the patient and monitoring during the imaging study, as they are most experienced in maintaining homeostasis in the neonate. This is especially the case for preterm infants, who have traditionally been managed with mechanical ventilation but more recently are being extubated at an earlier time point and ventilated using nasal continuous positive airway pressure (7). At UCSF, where MR imaging of prematurely born neonates is commonly performed, we use a prototype MR-compatible incubator with forced air heating and an infrared video system (8). Small windows in the walls of the incubator allow
monitoring equipment to be utilized while the patient is in the incubator. The child is not disturbed during the entire trip to and from the scanner (including the scan) except on the very rare occasions when problems occur. A similar system is now commercially available and reportedly works well, providing excellent images while allowing safety and close monitoring for the infant (9,10).
monitoring equipment to be utilized while the patient is in the incubator. The child is not disturbed during the entire trip to and from the scanner (including the scan) except on the very rare occasions when problems occur. A similar system is now commercially available and reportedly works well, providing excellent images while allowing safety and close monitoring for the infant (9,10).
We have found that when the baby is minimally disturbed in the incubator, we can perform MRI without sedation in as many as 70% of premature neonates; this should be attempted before sedation is administered (11). If MR imaging of premature infants is uncommonly performed at an institution and an MR-compatible incubator is not cost-effective, the infant may be wrapped in prewarmed towels and an air bag that is warmed to body temperature. Alternatively, chemical “blankets” containing mixtures of chemicals that maintain a temperature of 37°C when mixed can be used to maintain body heat beneath the airbag. A stockinet hat may be used to prevent heat loss from the head. Earplugs and earmuffs (we use both) reduce noise exposure and further reduce heat loss. Vital signs in these infants are monitored during both transportation and in the magnet. The child should be disturbed as little as possible (11).
Sedation
In modern clinical practice, sedation is rarely an issue for ultrasound or CT. Ultrasound is inherently “hands-on,” and excellent images can usually be obtained by targeted imaging and light restraints to movement. For CT, multidetector CT (MDCT) technology has significantly accelerated acquisition times, such that fewer than 1.5% of patients now require sedation with this modality (12,13). The use of soft restraints, although not usually necessary with MDCT, can further limit the movement of younger children to achieve satisfactory image quality.
The issue of sedation gains importance when considering MRI in young children. When children are unable to remain still, motion artifacts are likely to obscure important information and render exams clinically nondiagnostic. Sedation can be essential to achieve sufficient quality for diagnosis. At the same time, there is an increasing focus on the risks of sedation in the pediatric population and, as a result, a general decline in the use of sedation over the past decade. Currently, we use sedation only when infants need a rather extensive MRI (with multiple sequences), such as preoperative for suspected tumor or metabolic disease, and the child has been unable to lie sufficiently still to allow an adequate exam (11). Although several animal and in vitro studies have demonstrated immediate and in some cases longer-lasting or even permanent neuroanatomical and functional effects of common sedatives, clinical studies have been more reassuring, and the larger-scale studies that have been performed have not shown adverse effects of anesthetic exposure in terms of neurodevelopmental outcome. Nevertheless, the potential for neurotoxic effects of anesthesia led the U.S. Food and Drug Administration to issue a “Drug Safety Communication” in December 2016, stating that anesthesia for longer than 3 hours or repeated use of anesthetics in pregnant mothers or children less than 3 years of age “may affect the development of children’s brains.”
It is important to recognize that in most parts of the world (Europe, Asia, and South America as examples), the risk of sedation is considered to be much less than the risks associated with underlying injuries and diseases for which MRI is being performed, and sedation is used routinely in neonates, infants, and young children. Given its declining use, however, sedation is not covered in this chapter in the same detail that it has been in prior editions of this text. When sedation is necessary, the practitioner should be familiar with the guidelines published by the American Academy of Pediatrics (14,15), the American Society of Anesthesiologists (16), and the American College of Radiology (17). Again, at our institution, sedation in neonates has been largely supplanted by the feed-and-swaddle technique, which induces natural sleep and can usually be accomplished without any sedatives, occasionally supplemented with small amounts of morphine and/or sodium pentobarbital (11); however, this technique limits imaging to 4 or 5 relatively short sequences (11). When MRI is critical to diagnosis that will significantly change management, we rely upon the expertise of our pediatric anesthesiologists for longer examinations. In young children, we have also found that using MR-compatible video projection goggles and sound systems to display movies or videos during examinations suffices to distract these patients sufficiently to achieve a diagnostic examination. Age-appropriate scanner settings and antecedent education using child life specialists and MRI mock-ups to prepare children can also significantly reduce the need for sedation in this age group.
Monitoring
The American Academy of Pediatrics and the American Society of Anesthesiologists recommend that heart and respiratory rate, blood pressure, and arterial oxygen saturation be monitored in all sedated infants and children (14,15,16).
Monitoring a patient in the CT suite is relatively simple. A patient undergoing MRI is more difficult to monitor because of the safety issues and because biomonitoring devices (a) may not work properly in the magnetic environment and (b) may cause distortion of the magnetic field, especially within the narrow confines of the bore of the magnet. Monitoring must be performed using equipment composed of diamagnetic metals (e.g., aluminum) and/or plastics or composites. Several MRI-compatible monitoring devices are commercially available and are the best option for patient safety and optimal imaging at the present time. However, in the absence of MRI-compatible monitoring equipment, other techniques may be used. A plastic stethoscope with very long tubing may be taped to the patient’s chest so that heart rate can be monitored from outside the bore of the magnet.
Electrocardiogram leads, if necessary, may be run underneath the patient and as far away as possible from the body part being imaged. A CO2-sensitive apnea monitor connected by long, small caliber tubing to the patient in the scanner provides visual display of respiration and audio and visual alarms during apnea episodes without affecting image quality. Disposable, pediatric-size nasal cannulae are available. Fortunately, pediatric-sized MR-compatible monitoring equipment is now available from many manufacturers. If the practitioner is performing MR examinations on a large number of sedated children, this equipment is well worth the investment.
Although some lower field strength magnets and shielding configurations will allow life-support equipment to be in close proximity to the patient, most high-field MR suites require significant modifications to achieve intense, close-in electronic monitoring. MR-compatible pediatric ventilators are now commercially available and in broader use; in their absence, respirator-dependent patients must be manually ventilated. In extreme circumstances, significant respiratory or hemodynamic instability may require that the physician or nurse crawl into the bore of the scanner and observe the child from this extremely uncomfortable position to ensure the safety of the patient through the duration of the examination.
A brief mention should be made of the impact that monitoring and sedation can have on image quality. One example of this is T2 FLAIR hyperintensity in cerebrospinal fluid (CSF) that is seen in patients who are administered supplemental oxygen in high concentration during MRI. In this situation, oxygen in the blood diffuses across a
concentration gradient into the CSF, effectively increasing its oxygen concentration. With its two unpaired electrons, oxygen is weakly paramagnetic and effectively shortens the T1 relaxation time of the CSF, often to a sufficient degree to compromise the CSF nulling pulse and cause abnormal hyperintensity within CSF on T2 FLAIR images that might otherwise be mistaken for hemorrhage, pus, or carcinomatosis. Unlike these CSF diseases, oxygen-related CSF hyperintensity on T2 FLAIR images is typically diffuse and involves the entirety of the CSF rather than concentrated locally in sulci or cisterns. The effect is further potentiated by certain anesthetics, notably propofol. Thus, if CSF hyperintensity is seen in the cisterns on T2 FLAIR sequences, any sedation and the concentration of supplemental oxygen must be researched before the scan is interpreted as abnormal. Using lower concentrations of supplemental oxygen (50%-60% works well) eliminates this artifact (18,19).
concentration gradient into the CSF, effectively increasing its oxygen concentration. With its two unpaired electrons, oxygen is weakly paramagnetic and effectively shortens the T1 relaxation time of the CSF, often to a sufficient degree to compromise the CSF nulling pulse and cause abnormal hyperintensity within CSF on T2 FLAIR images that might otherwise be mistaken for hemorrhage, pus, or carcinomatosis. Unlike these CSF diseases, oxygen-related CSF hyperintensity on T2 FLAIR images is typically diffuse and involves the entirety of the CSF rather than concentrated locally in sulci or cisterns. The effect is further potentiated by certain anesthetics, notably propofol. Thus, if CSF hyperintensity is seen in the cisterns on T2 FLAIR sequences, any sedation and the concentration of supplemental oxygen must be researched before the scan is interpreted as abnormal. Using lower concentrations of supplemental oxygen (50%-60% works well) eliminates this artifact (18,19).
Contrast Media
The indications for administration of intravenous contrast media in CT for neuroradiology are declining and limited now primarily to angiographic studies and studies outside of the brain, for example in the head and/or neck. Occasionally, when a noncontrast CT of the head reveals an acute abnormality, such as subdural empyema, the decision to administer contrast can be made when MR cannot be obtained in a timely fashion in order to obtain an accurate diagnosis or more completely delineate an abnormality. However, very little information is gained, in general, by administration of CT contrast unless a vascular lesion is suspected (20).
When CT is necessary, nonionic, iso-osmolar, or low-osmolar contrast media have been shown to be safer and less uncomfortable for the patient. The exact type of iodinated contrast is not important, as long as the concentration of iodine is approximately 300 mg/mL. The recommended dose is 3 mL/kg of body weight up to a total dose of 120 mL. In view of their rapid heart rate, children should be scanned as soon as possible after contrast has been administered. Thankfully, adverse reactions to iodinated contrasts are rare in the pediatric population and exceptionally infrequent in the youngest patients (21). Acute reactions are most common in children weighing 24 to 40 kg. Asthma and previous reactions to contrast medium are risk factors for acute reactions (21).
For almost all indications, MRI can be performed without intravenous contrast with no compromise in examination quality. There are several situations, however, in which intravenous gadolinium-based contrast agents (GBCAs) can improve diagnostic accuracy and lesion detection. These include some MR angiographic and perfusion techniques and for disorders such as primary and metastatic brain and spine tumor, infection (abscess, empyema, cerebritis, meningitis), and certain neurocutaneous disorders (neurocutaneous melanosis, neurofibromatosis type II, Sturge-Weber syndrome) (22,23). Administration of contrast to infants or children undergoing evaluation for developmental delay or epilepsy is unlikely to be of benefit unless a space-occupying lesion is identified or a cutaneous lesion suggesting one of the neurocutaneous disorders mentioned above is present. Similarly, no advantage is gained by administering GBCAs to children with developmental malformations of the brain or spine, with the exception of dermal sinus tracts.
From the standpoint of diagnostic efficacy, there does not appear to be a significant difference among commercially available paramagnetic contrasts. All are given intravenously; for most neurologic applications, the standard dose is 0.1 mmol/kg. It is important to note that agents now exist in different concentrations, such that volume of contrast administered for the same dose depends upon the GBCA that is used. After infusion of contrast, any pulse sequence sensitive to T1 contrast will be sensitized to the effects of the agent. GBCAs also have a T2*shortening effect that is exploited for some MR-based cerebral perfusion techniques. Fat suppression may be useful if meningeal disease is suspected (24) or if suspected pathology lies in the orbits or neck, where fat may obscure the enhancing lesion. Similarly, postgadolinium FLAIR imaging can be particularly useful in the assessment for abnormalities of the CSF.
Once widely administered in the setting of impaired renal function, GBCAs are now known to be associated with nephrogenic systemic fibrosis (NSF) in patients with renal insufficiency (25,26). This rare but serious systemic disorder causes fibrosis of the skin and other tissues, thereby leading to considerable morbidity or even death. All reported cases in children (27,28) have occurred in patients with estimate glomerular filtration rate (eGFR) less than 30 mL/min. A proinflammatory state (systemic infection, limb or major tissue injury, recent transplant surgery, or thrombosis) may further increase the risk of developing this complication.
More recently, it has been noted that exposure to multiple doses of some GBCAs may cause increased T1 signal intensity in certain brain structures, such as the globus pallidus and deep cerebellar nuclei (29,30,31). Although the mechanism underlying this phenomenon is still under investigation, brain autopsy studies documenting retained gadolinium within the endothelial walls and interstitium indicate that the metal likely becomes dissociated from the chelate to which it is bound before it is eliminated from the bloodstream. Gadolinium deposition has been most convincingly described in patients receiving linear GBCAs. However, gadolinium deposition has also been reported with macrocyclic GBCAs and in our opinion likely occurs to varying degrees with all GBCAs. Pathological studies have not shown evidence of neuronal injury, and there are no known clinical conditions associated with these findings. Nevertheless, this “brain stain” from gadolinium underscores the need to carefully consider whether gadolinium contrast is necessary for diagnosis when performing MRI in children. If used, the macrocyclic agents are preferable, as the gadolinium ion may be more tightly chelated (32).
With the recognition of the relationship between gadolinium and NSF and the observation of gadolinium deposition, there is no longer justification for administering contrast to every sedated patient to avoid the need to resedate the child for a repeated scan with contrast. When gadolinium is necessary, screening for diminished GFR is essential and the relative risks and benefits in patients with renal failure should be considered in consultation with referring physicians. The reader should be aware, however, that as of the date of this revision, there have been no new reported cases of NSF since 2009. Limited use of GBCAs in patients with low GFR, the adoption of more stable macrocyclic agents, and restricting the maximum dose to 0.1 mmol/kg together most likely account for the decline in the incidence of NSF.
Other reported side effects of gadolinium are exceptionally rare. Allergic reactions are even less common with GBCAs than they are with iodinated contrast. In one study of 13,344 examinations in which gadolinium was given to children, the rate of acute allergic reaction was 0.04%. With respect to its use in pregnancy, intravenous GBCAs cross into the placenta and are excreted into the amniotic fluid. In high and repeated doses, intravenous gadolinium is teratogenic in animal studies (33). Although no similar effects have been observed in human studies of teratogenicity, it has been our practice to avoid the use of gadolinium in pregnancy except when absolutely essential. There are no indications for which maternal paramagnetic contrast agent administration is necessary for fetal MRI.
Another class of paramagnetic contrast agent that may be used in some situations are the ultrasmall superparamagnetic iron oxide
(USPIO) compounds. Ferumoxytol is increasingly being reported as an alternative to GBCAs for vascular imaging, particularly in patients with renal insufficiency. This medication, first investigated over a decade ago, enjoys much stronger T1 relaxation effects than do GBCAs. Moreover, because of its size and carbohydrate coating, the agent has a long intravascular residence of more than 12 hours and can be used to achieve higher-quality vascular imaging than the GBCAs. However, ferumoxytol is used “off-label” as a contrast agent as the primary indication for the medication is iron deficiency anemia in chronic kidney disease. Moreover, the FDA issued a boxed warning for the medication in 2015, citing the incidence of severe anaphylactic reactions in a small number of patients, recommending that it should be used only for patients who require intravenous iron therapy. Nevertheless, ferumoxytol is still useful as a contrast agent in a subgroup of patients and occasionally used at academic medical centers (34), particularly for the study of vascular disease (35,36).
(USPIO) compounds. Ferumoxytol is increasingly being reported as an alternative to GBCAs for vascular imaging, particularly in patients with renal insufficiency. This medication, first investigated over a decade ago, enjoys much stronger T1 relaxation effects than do GBCAs. Moreover, because of its size and carbohydrate coating, the agent has a long intravascular residence of more than 12 hours and can be used to achieve higher-quality vascular imaging than the GBCAs. However, ferumoxytol is used “off-label” as a contrast agent as the primary indication for the medication is iron deficiency anemia in chronic kidney disease. Moreover, the FDA issued a boxed warning for the medication in 2015, citing the incidence of severe anaphylactic reactions in a small number of patients, recommending that it should be used only for patients who require intravenous iron therapy. Nevertheless, ferumoxytol is still useful as a contrast agent in a subgroup of patients and occasionally used at academic medical centers (34), particularly for the study of vascular disease (35,36).
 Ultrasound
UltrasoundUltrasound is always the first study of choice in the fetus. It is also nearly always the first in neonates, as it is noninvasive, inexpensive, and portable (can be performed at the bedside); requires no sedation; and produces no ionizing radiation. The modality has improved in recent years with more widespread availability of high-frequency transducers, the introduction of high-bandwidth tissue harmonic imaging techniques, and the use of multiple acoustic windows, to the point where much information can be gathered about most regions of the brain from a high-quality ultrasound examination. Indeed, measurements of brain structures with ultrasound and MRI are nearly identical (37), with small observed differences (mainly cortical thickness and interhemispheric fissure size) most likely due to inability to accurately differentiate the cortex from the overlying leptomeninges on ultrasound. The peripheral aspects of the brain may also be difficult to visualize, particularly when small fontanelles limit the size of the acoustic window; this limitation has been markedly reduced, however, with current equipment. After the first few months of life, however, as the brain grows and the sutures/fontanelles close, ultrasound rapidly becomes less useful for brain and spine, and MR becomes the imaging modality of choice.
Meticulous technique on the part of well-trained sonographers must be used to optimize ultrasound studies. Imaging should always be performed using multiple transducers functioning at variable frequencies. Vector, curved, and linear array transducers should all be used. Resolution and depth penetration can be optimized by adjusting the frequencies (between 8 and 17 MHz) and the focal zone of the ultrasound beam. When the brain is thus analyzed via the anterior and posterior fontanelles and the temporal, mastoid, and occipital synchondroses, all regions of the brain (central and peripheral) can be seen well. Abnormalities will be shown best if images are acquired in sagittal, parasagittal, coronal, and axial planes. Real-time images allow an excellent appreciation of subtle changes in echogenicity and have, essentially, replaced static images at UCSF. Major arteries and veins should be assessed by Doppler techniques, looking for peak systolic velocities, end-diastolic velocities, and resistive indices.
 Computed Tomography
Computed TomographyMedical radiation is a particularly important consideration in pediatric radiology, as younger patients have a greater chance of developing radiation-associated diseases (38,39,40) and, possibly, developmental impairment (41). Overutilization of CT in the past decade (2000-2010) among the entire US population, including pediatric patients, and the variability in scan parameters for CT (42) resulted in an increased focus on the need to consider alternative modalities first (43). When CT is necessary, one must also consider whether dose reduction techniques (44,45) should be used, recognizing that the diagnostic quality of the imaging study may suffer when image contrast to noise is reduced by dose reduction (46,47). Institutional approaches based on systematic consideration of clinical indications and prior history of exposure to ionizing radiation may help to increase the consistency with which specific parameters are used for pediatric CT scanning and to reduce overall radiation exposure (48).
The indications for the study and scan parameters should be weighed together. For example, when a child has sustained acute head trauma, the concern is mainly for fractures, pneumocephalus, or a space-occupying hematoma that may alter emergent management. The goal in this scenario is rapid diagnosis and not necessarily highresolution evaluation of the brain parenchyma; therefore, low-dose CT is adequate for most cases. If indicated, an MR can be acquired when the patient is more stable to better evaluate the extent of any brain injury. In contradistinction, in the acutely encephalopathic child with new neurological signs or symptoms, our approach is to get an MR on a timely basis. If MR is contraindicated or unavailable, we obtain a standard-dose CT scan of good quality in order to avoid repeating the scan or necessitating another type of scan. When arterial or venous thrombosis is suspected, we will usually obtain an magnetic resonance angiography (MRA) or magnetic resonance venography (MRV). If questions arise due to saturation effects resulting from complex flow, contrast-enhanced MRA or MRV will almost always answer them. Very rarely, CT angiography (CTA) may be necessary, as it is less affected by artifacts and can be performed rapidly and without sedation. However, the scan should be limited to specific regions of concern, and exposure of the eyes, thyroid, and breasts should be minimized.
When patients have conditions that will require a number of imaging studies over many years, the imaging technique of choice requires considerable thought. For example, patients with hydrocephalus will often have multiple, possibly dozens, of scans during childhood, and the accumulated radiation dose can become significant (49). If the purpose of the scan is only to check ventricular size after placement or revision of a ventricular catheter in a child with known hydrocephalus, a high-dose technique is not necessary; instead, one should consider a CT technique using lower tube voltage or tube current. By reducing CT dose from 220 to 80 mA, one study showed a reduction in radiation dose of 63% while maintaining diagnostically acceptable images (50); as noted above, however, the reduction of signal to noise that accompanies the dose reduction severely limits assessment of brain parenchyma. At UCSF, we have chosen to acquire a fast MR sequence as our initial study if a child is too old for ultrasound. Initially, we use single-shot fast spin echo (ssFSE) or half-Fourier acquisition single-shot turbo spin echo (HASTE), or a periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) sequence, thereby eliminating ionizing radiation altogether. In the age of magnetically adjustable pressure valves, however, the use of MR in a child with shunted hydrocephalus requires a visit to the neurosurgeon after the scan to readjust the valve.
If, after careful consideration, CT is considered necessary based on the indication and the condition of the patient, the study should be performed with the lowest possible dose that allows a diagnostic quality scan. Some recent reviews of methods for calculating and reducing dose for patients of all ages elegantly describe how to accomplish this (44,45). These methods should be familiar to all physicians involved in imaging. The techniques for scanning older children with CT are identical to those for scanning adults. Axial images are obtained using ≤3-mm slice thickness; an eye shield may be used, and the plane of section should be chosen to minimize exposure to the eyes.
As CT scanning has become more rapid, little time penalty is paid for relatively thin slice profiles, and significant additional information may be acquired, especially in the small heads of infants and young children. However, a price is paid in that the signal to noise diminishes with decreasing slice thickness and this must be compensated with increased tube voltage or tube current, which increases dose. To mitigate that issue, the thinner sections can be reformatted as thicker (4-5 mm) sections, improving signal to noise and allowing reformation of thinner sections (e.g., when looking for fractures) if desired (44). We typically acquire sequential scans rather than using spiral acquisition for head studies, as signal to noise is improved, artifacts are avoided, and there is little time penalty (51). If coronal images are needed, strong consideration should be given to reformatting axial images; this saves the added radiation of an extra sequence. In such cases, spiral acquisition may be desirable. A low pitch (<1) should be used if higher resolution is needed; reducing tube current settings can ameliorate any increase in radiation dose. If spiral CT is not available, the higher resolution may be obtained via the use of direct coronal images; the nonsedated patient may be examined in the prone or supine positions, but coronal scans of sedated patients are best performed in the supine position to avoid compromising the airway. Ideally, the plane of scanning should be perpendicular to the planum sphenoidale.
Another special situation in pediatric CT imaging in which techniques can be modified is in the scanning of patients with craniofacial anomalies or craniosynostosis. These patients should be scanned using ≤2.5-mm slice thickness. Thicker slices allow too much averaging and obscure detail. Reconstruction algorithms that give high-detail bone resolution should be used to evaluate the cranium; they give better images with a lower radiation dose. If no MR scan will be obtained, discuss with the referring surgeon the use of a soft tissue algorithm to assess the underlying brain for abnormality; the unnecessary data can then be discarded during the process of reconstructing 3D reformations. Software that allows three-dimensional surface- and volumerendered reconstructions of the bones of the face and skull is available from most manufacturers; these have significant value in assessing suture patency and in the planning of reconstructive surgery.
Because the newborn brain has a very high water content, proper windowing of the CT scan is essential for optimal analysis of brain abnormalities. In general, CT images of the newborn brain should be reviewed with a window of approximately 60 and level of approximately 20. Use of normal adult brain windows will result in pathology being missed.
Table 1-1 Sequences Used for Evaluation of Brain Anatomy in Most Children | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 MRI: Anatomic Imaging
MRI: Anatomic ImagingMR protocols for imaging at UCSF are categorized by anatomic location and by indication, so that disease-relevant abnormalities can be consistently identified and characterized. Although vendor- and techniquerelated variability is to some degree unavoidable across different scanners, this approach to protocol development helps to standardize acquisition techniques for consistent image quality across the multiple MR systems used for pediatric patients in our practice and to facilitate longitudinal follow-up when monitoring changes in disease over time. Each protocol specifies the field of view (FOV), matrix size, and slice thickness for the desired spatial resolution and anatomic coverage. The protocol also specifies appropriate scanning parameters, including flip angle (flip), repetition time (TR), echo time (TE), number of signal averages (NEX), readout bandwidth (BW), and, if appropriate, the echo train length (ETL) and inversion time (TI). These technical parameters may differ slightly for different scanner vendors and field strengths. Finally, specifics regarding fat and flow suppression and which reformats should be routinely generated are also included in protocols. The fundamental elements of these protocols are outlined below by body part, in a similar fashion to how they are organized in our practice.
Brain
The primary pulse sequences used for the evaluation of brain structure are summarized in Table 1-1. T1- and T2-weighted images are obtained using gradient-recalled echo (GRE) and fast spin-echo (FSE) techniques. Although it sometimes provides the best contrast, singleecho spin-echo acquisition requires longer scan times and is now less commonly used. Given its short imaging time and sensitivity to a broad range of diseases, diffusion-weighted imaging (DWI) is included in almost all brain protocols. Although state-of-the-art DWI is also capable of depicting brain anatomy in high detail, we defer discussion of this technique until the next section, which focuses on physiologic and metabolic MR techniques. In our practice at UCSF, susceptibilitysensitive (T2*-weighted) imaging is also included for most indications.
High-quality T1-weighted imaging is essential for accurate identification of structural brain abnormalities. Volumetric 3D T1 GRE techniques such as spoiled gradient echo (SPGR) and magnetizationprepared rapid gradient echo (MP-RAGE) (52) are favored as, unlike conventional 2D FSE, the resulting images can be reformatted in any
plane, exhibit strong contrast between gray and white matter, and allow high spatial resolution to be achieved within clinically feasible scan times using short TR and TE (typically 35 ms for TR and minimum TE). Using a sagittal prescription oriented such that the longest (craniocaudal) axis is the readout direction, the total acquisition time for this sequence is approximately 5 minutes for 3 T scanners. The FOV and matrix size for 3D T1 imaging in children should be set so that the voxel size is no greater than 1 × 1 × 1 mm.
plane, exhibit strong contrast between gray and white matter, and allow high spatial resolution to be achieved within clinically feasible scan times using short TR and TE (typically 35 ms for TR and minimum TE). Using a sagittal prescription oriented such that the longest (craniocaudal) axis is the readout direction, the total acquisition time for this sequence is approximately 5 minutes for 3 T scanners. The FOV and matrix size for 3D T1 imaging in children should be set so that the voxel size is no greater than 1 × 1 × 1 mm.
When contrast-enhanced T1-weighted imaging is necessary, we use slightly different parameters with 3D T1 GRE sequences in order to heighten sensitivity to the T1-shortening effects of gadolinium. If volumetric images cannot be acquired, T1 fluid-attenuated inversion recovery (FLAIR) or FSE sequences can be used instead. Although T1 FLAIR gives better T1 information than do FSE sequences, a T1 FSE with TR/TE = 600/minimum sequence can be routinely used for T1-weighted images on 1.5 T scanners.
Although 3D T1 GRE acquisition is useful for identifying small lesions given its superior spatial resolution, T1 FSE is the sequence of choice when paramagnetic contrast is to be administered. Use of this imaging sequence produces excellent images with greater enhancement contrast; moreover, the imaging time is shorter than with most inversion recovery sequences, resulting in less motion artifact. Spin-echo T1 images do not work as well at 3 T due to the prolongation of T1 relaxation times, however. Therefore, T1 FLAIR (usually using short TE in the range of 20 ms and TI = 750 ms) sequences are used; these give excellent T1 weighting but do not depict contrast enhancement as well as do FSE or 3D T1 GRE images.
Anatomic detail can be visualized well using 3D T1 by reformatting the acquired images to the optimal planes that depict different structures. For example, we find that sagittal images are best for evaluating the corpus callosum, pituitary gland, hypothalamus, and cerebellar vermis, common locations of pediatric brain tumors. They are also excellent for assessing the lateral convexities of the cerebral hemispheres, especially around the sylvian fissures. Ventricular morphology, the septum pellucidum and brain stem are seen well on axial images, and the cerebellar hemispheres, temporal lobes, skull base, and commissural white matter tracts are well evaluated on coronal images. 3D technique is very valuable in the imaging of tumors, where the relationship of the mass to the surrounding brain and dura is of great importance to the neurosurgeon. As 3D T1 acquisition techniques are increasingly standardized across vendors, this sequence is also gaining importance for its role in computational structural analysis, automated diagnosis, and statistical comparisons with atlas-based reference data (53,54).
For T2-weighted imaging, the ETL for FSE acquisition should be kept low (≤4) in order to achieve satisfactory contrast without sacrificing spatial resolution. With parallel imaging, it is now possible to obtain volumetric T2-weighted images using variable flip angle FSE techniques (CUBE, SPACE, VISTA). Although these enjoy high spatial resolution, we have found that the tissue contrast that results with these methods is not yet sufficient to use for routine imaging protocols in children. Instead, we most often employ traditional multislice (2D) FSE acquisition with 4- to 5-mm slices (2-2.5-mm gap). Dual short and long echo FSE is used, with TR/TE = 3000/60, 120 ms in infants less than 12 months old and TR/TE = 2500/30, 80 ms otherwise. More heavily T2-weighted sequences are recommended in the first year of age, as the water content of the brain in young children is considerably higher than in older children and adults (55,56). In this patient group, we use TR/TE = 3000/120 ms. Others have used a dual-echo short tau inversion recovery (STIR) sequence with TR/TE/TI = 5400/128/130 ms (23), although in our experience noise in STIR images can sometimes make them difficult to interpret. Variable flip angle 3D FSE, although it does not enjoy the same level of tissue contrast, can be useful to supplement 2D FSE in patients with epilepsy to identify sulcal and gyral morphological abnormalities.
Some authors have advocated the use of T2 FLAIR to look for regions of abnormal T2 prolongation. In our experience and that of others (57), FLAIR images are not sensitive to cerebral pathology in neonates or infants, but are useful in older children in whom myelination is complete or nearly complete. We do not use FLAIR as a primary sequence in infants, but we do sometimes use it as a secondary sequence for lesion characterization. In children beyond the age of 2 years, when myelination is nearly complete, FLAIR becomes a part of our routine protocol because of its high sensitivity for subtle supratentorial lesions, particularly in the cerebral cortex and periventricular white matter. Sargent and Poskitt found that FLAIR is complementary to T2-weighted infants in children (58). They found FSE T2 FLAIR to have better CSF nulling and better gray matter-white matter differentiation than echo-planar FLAIR.
3D T2 FLAIR using CUBE, SPACE, or VISTA (depending on the scanner manufacturer) is our preferred method for T2 FLAIR acquisition. These have the benefit of very high spatial resolution of 1 to 2 mm and relatively short imaging times of 5 to 6 minutes and like volumetric gradient echo T1 images can be reformatted in any plane. Similar to all 3D techniques, longer imaging times render 3D T2 FLAIR sequences more susceptible to artifacts from patient motion, and in our experience, the tissue contrast of such sequences does not always compare favorably to 2D sequences.
It should be emphasized that in patients less than 18 months of age, both T1- and T2-weighted images are necessary for accurate interpretation. Brain maturation is evaluated best by T1-weighted images from birth to 6 months of age. However, from 6 to 8 months of age until approximately 24 months of age (at which time the brain is essentially mature by MR standards), T2-weighted images are more useful for assessing myelination and brain maturity (see Chapter 2 for more details on timing of brain maturation). During the process of white matter maturation, the cerebral cortex and subcortical white matter of the brain become isointense for a variable period of time on MR images; this loss of intrinsic contrast obscures structural detail. Therefore, during the first 8 months of life (while the white matter is maturing on T1-weighted images), T2-weighted images are required to see the details of the gyral and sulcal patterns. Similarly, as white matter matures on T2-weighted images between 8 and 24 months of age, T1-weighted images are essential for evaluation of structural abnormalities.
A significant drawback of T2-weighted and FLAIR sequences is longer imaging times when compared to T1-weighted imaging. Some extremely fast MR techniques can be used in selected cases without sedation. These include the previously discussed ssFSE (59,60) and PROPELLER (61,62); both are most useful for gross assessments such as ventricular size in patients with hydrocephalus or follow-up of extraparenchymal fluid collections. PROPELLER has the advantage of better contrast to noise, more flexible contrast, and the ability to compensate for motion by retrospectively correcting the acquisitions (called “blades”), but has the disadvantage of slightly longer acquisition times (61). When we do use PROPELLER FSE, the parameters applied as TR/TE = 4000/83 ms, NEX = 2, FOV = 24 cm, matrix = 224 × 224, and 4- to 5-mm slice thickness. In our experience, however, tissue contrast is not as good in PROPELLER sequences as in conventional spin-echo or STIR sequences. Our routine rapid acquisition MRI protocol acquires axial DWI images and then 2D HASTE images in orthogonal axial, sagittal, and coronal planes and does not use PROPELLER. For HASTE, we use TR/TE = 20,000/90 ms, NEX = 0.5, FOV = 24 cm, matrix = 256 × 256, and 4-mm slice thickness. As noted above, we have found that this short protocol can be effectively used to evaluate ventricular size in children with suspected shunt dysfunction.
A number of sequences have been developed to exploit contrast related to magnetic susceptibility, including T2*-weighted GRE, susceptibilityweighted imaging (SWI), and susceptibility-weighted angiography (SWAN). They share in common the use of short TR, long TE (25-50 ms) GRE acquisition, which sensitizes images to T2* contrast. The techniques differ in their use of 2D or 3D encoding, the application of flow compensation gradients to reduce flow artifacts and the approaches used to process the complex-valued MR measurements, which consist of two values—one representing magnitude and the other phase—for each voxel in an image.
The minute distortions in the magnetic field that are induced by paramagnetic or diamagnetic tissue components give rise to signal loss, or “blooming,” on magnitude images. These are often useful to identify for areas of old hemorrhage, as in suspected trauma or vascular malformations (63,64,65). Beyond infancy, these images may sometimes also provide the only clue on MRI that calcification is present, for example within a brain tumor or as the result of congenital infection.
The phase component is ignored for most sequences, and only the magnitude component is used to construct the image. However, image phase in T2*-weighted images is linearly related to tissue magnetic susceptibility and can be exploited to visualize this information. Paramagnetic compounds such as hemosiderin or ferritin within hemorrhage or deoxyhemoglobin within veins cause a positive phase shift, whereas diamagnetic compounds such as calcium induce a negative phase shift. With SWI, phase information is added to the reconstructed image in order to heighten sensitivity to susceptibility effects. Phase images can also be viewed directly to help determine whether tissue is paramagnetic or diamagnetic and thereby differentiate between iron- and calcium-containing compounds. As 3D techniques, SWI and SWAN can take longer to acquire than conventional T2*-weighted GRE, but satisfactory resolution can usually be obtained in under 5 minutes. New versions that use echo-planar acquisition are being developed that should shorten the acquisition time (63).
It should be emphasized that higher field strength increases sensitivity to susceptibility contrast, such that 3 T is more likely to reveal the presence of these tissue components than 1.5 T. 7 T MRI scanners, one of which has received conditional FDA approval at the time this chapter was written, permit greater susceptibility contrast and for this reason are likely to gain traction for clinical evaluation in certain indications in the near future.
Vascular Imaging
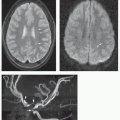
Both CTA and MRA are accurate and easy; both show the vessels of the neck, the dural venous sinuses, and circle of Willis quite well. However, considering the considerable dose of ionizing radiation from CTA, MRA is considered a better initial study in children, even when sedation is necessary. At UCSF, we only perform CTA if the necessary diagnostic information cannot be obtained by other methods.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree