Fig. 31.1
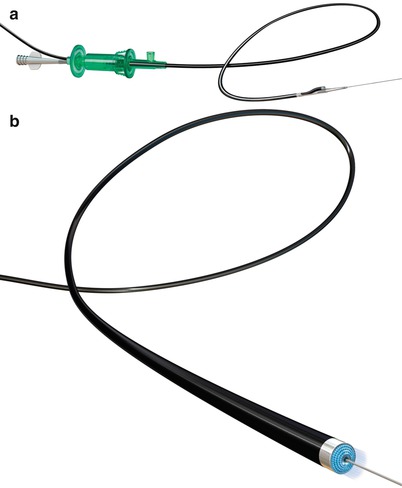
(a) FRONTRUNNER XP Catheter engaging a peripheral chronic total occlusion lesion (b) FRONTRUNNER XP blunt microdissection within chronic total occlusion segment
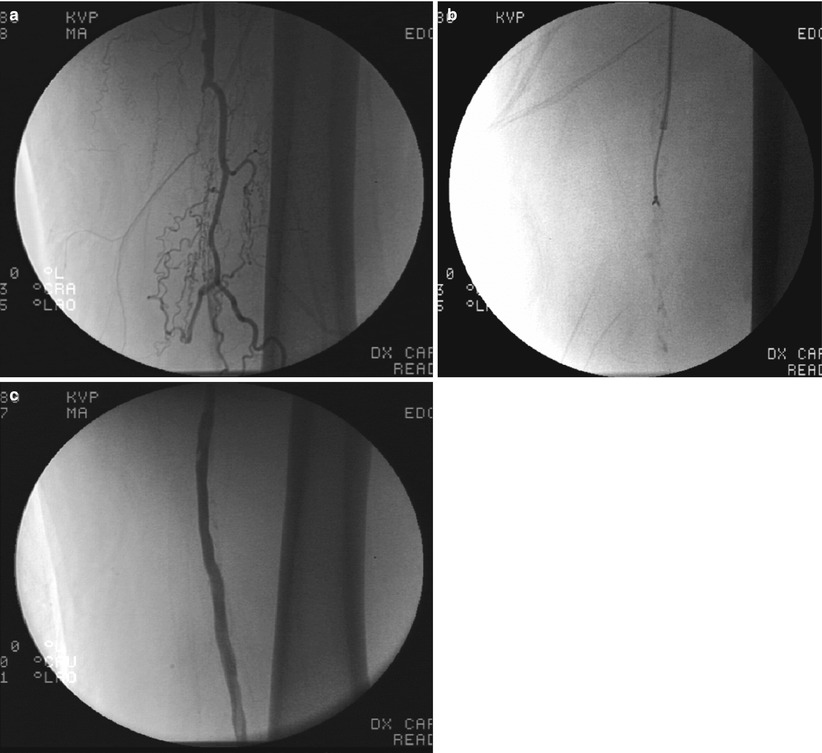
Fig. 31.2
(a) Chronic total occlusion of superficial femoral artery. (b) FRONTRUNNER XP blunt microdissection within chronic total occlusion. (c) Final post stent angiogram
CROSSER Catheter
The CROSSER chronic total occlusion recanalization system (Bard Peripheral Vascular) is an innovative device that uses high-frequency mechanical vibrations (20,000 cycles per second to a depth of 20 μm) propagated through a nitinol core wire to a stainless steel tip (Fig. 31.3). The system is comprised of an electronic CROSSER generator, foot switch, high-frequency transducer, the FlowMate Injector, and CROSSER catheter. The generator applies AC current to the piezoelectric crystals in the transducer, which then converts, amplifies, and transmits this energy to the catheter tip. The vibrational mechanical impact and cavitational effects result in penetration of the occluded artery. The catheter is mounted on a standard 0.014-in. guidewire and is compatible with a 5F guiding catheter.
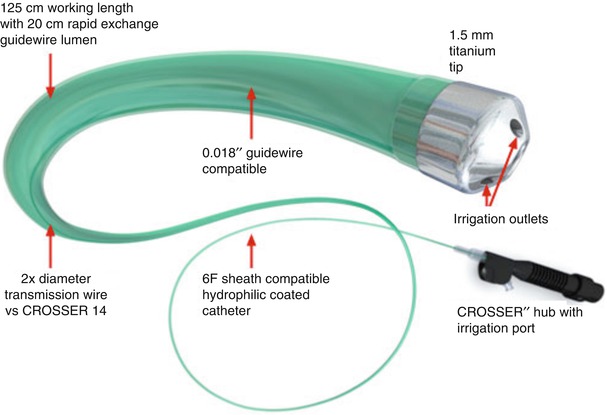
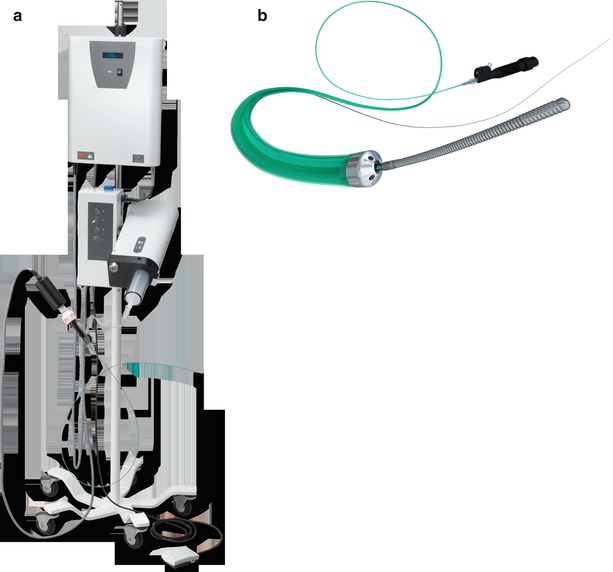


Fig. 31.3
(a and b) CROSSER chronic total occlusion recanalization system (©2012 C. R. Bard, Inc. Used with permission)
For the typical procedure with an over-the-wire catheter, a 0.014 guidewire is advanced to the proximal cap of the chronic total occlusion. The CROSSER is then passed over the guidewire until it comes in contact with the proximal cap. The guidewire is withdrawn and the device is activated with the foot pedal and slowly advanced into the occlusion. Gentle forward pressure is applied to cross the lesion, allowing the device to find its way. Once crossing of the lesion is confirmed by angiography, the guidewire is advanced into the distal lumen. Use of chilled saline for irrigation is essential to avoid excessive heating of the device. Guidewires with polymer-coated distal ends should not be used.
The basis for infrainguinal approval of the CROSSER device was the PATRIOT trial [13]. In this study, a technical success rate of 84 % was achieved in crossing peripheral CTOs. The average occlusion length was 117.5 mm, and 75 % of the lesions were moderate to severely calcified. Available case series demonstrated a success rate of 41–75 % in peripheral interventions with the CROSSER device [14, 15].
The TruePath Crossing Device
The TruePath device (Boston Scientific Corporation, Natick, MA) is designed to penetrate hard or calcified occlusions and create microdissection in CTOs to facilitate access to the distal true lumen (Fig. 31.4). It is a 0.018-in. wire with a diamond-coated tip that rotates at 13,000 rpm. A support catheter is used to advance the TruePath to the lesion. The device is then activated and advanced across the lesion. It has a built-in safety mechanism with audible and visual alerts if excessive resistance is encountered. The working length is 165 cm, which can be extended to 300 cm to allow for catheter exchanges. The tip may be bent up to 15° for added steerability. A support catheter is mandatory when using the TruePath device. A preshaped support catheter is preferred with this device. For added support, the device tip should not be extended 1 cm beyond the support catheter. Shaping the device tip may help with steerability through complex occlusions. Gentle forward pressure and slow device advancement is recommended.
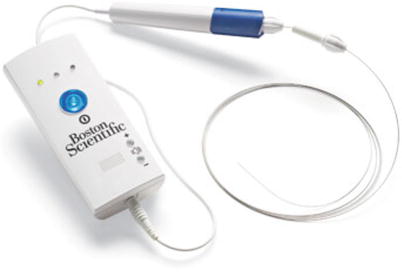

Fig. 31.4
TruePath catheter (Courtesy of Boston Scientific Corporation, Natick, MA)
TruePath was originally evaluated in the investigational device exemption ReOpen study in which a technical success rate of 80 % and primary safety end point rate of 98.8 % was achieved. The mean lesion length was 166 mm, the fluoroscopy time was 29 min, and the device activation time was 8.2 min.
Avinger Devices
The Wildcat, Kittycat, and Ocelot devices (Avinger, Inc., Redwood City, CA) utilize a corkscrew action to traverse CTOs. The Wildcat is a 0.035-in. catheter with a hydrophilic coating that facilitates tracking through complex lesions in the SFA. The rotatable tip assumes both passive (wedges in) and active (wedges out) configurations. The tip is initially advanced to the lesion and rotated in the passive mode. For more fibro-calcified lesions, the active mode may be used to traverse the CTO. The tip may be rotated manually or with a handheld motorized unit called the juicebox. The 0.014-in. versions of this device (Kittycat and Kittycat 2) provide smaller crossing profiles and longer working lengths for tight above-the-knee lesions or tibial vessels. The Ocelot device (Avinger, Inc.) is the latest addition to this family that incorporates real-time optical coherence tomography built into the catheter to guide intraluminal crossing (Fig. 31.5). The catheter tip may be rotated clockwise, counterclockwise, or a combination of the two with a gentle forward pressure. Counterclockwise rotation is less aggressive and should generally be tried first. The device should not be rotated in one direction for prolonged periods of time. Passive mode may be effective in softer occlusions and can be attempted before active mode. The active mode is more effective in fibrocalcific lesions. If significant resistance is encountered at the proximal or distal cap, only gentle forward pressure should be applied, allowing the device wedges to do the work. If the catheter becomes subintimal, it is withdrawn and additional attempts are made to reenter the true lumen or the tip of the catheter is deflected.
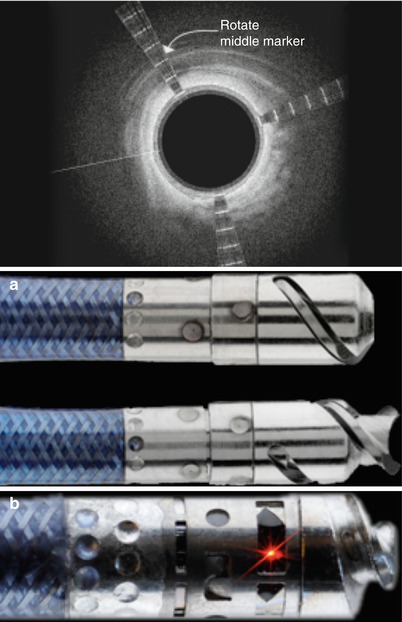

Fig. 31.5
(a) Ocelot device and (b) Optical coherence tomography orientation system (Courtesy of Avinger, Redwood City, CA)
Wildcat was evaluated in the CONNECT (Chronic Total Occlusion Crossing With the Wildcat Catheter) trial in which the technical success rate of crossing CTOs was 89.3 %, with a primary safety rate of >95 %. The average lesion length was 174 mm, and approximately half of the lesions were moderately calcified.
Viance and Enteer Peripheral CTO Crossing and Reentry Devices
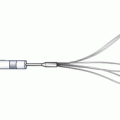
This is a novel peripheral CTO crossing system (Covidien) and has yet to be approved by the US Food and Drug Administration for peripheral vascular use (Figs. 31.6 and 31.7). The system is based on a coronary system called the CrossBoss and Stingray devices. It is composed of a crossing catheter that facilitates recanalization of the lesion and a reentry device to facilitate access to the distal true lumen, in case of subintimal tracking. The crossing catheter has a working length of 150 cm and tracks over a 0.014-in. guidewire. It has a coiled shaft and an atraumatic distal tip to prevent vessel exit. Using a torque device, the catheter is rapidly spun in either direction to facilitate advancement through the lesion. If the crossing catheter enters the subintimal plane, the reentry device is used to access the distal true lumen. This is the only reentry device designed for below-the-knee use. The reentry system consists of an orienting balloon catheter and a reentry guidewire. The balloon catheter has a 150-cm shaft length and is 0.018-in. wire compatible. The balloon is available in two sizes (3.75 × 20 mm and 2.75 × 20 mm) to facilitate reentry both above and below the knee. The balloon has a flat shape, which is designed to self-orient one of two 180° offset exit ports toward the vessel true lumen upon low-pressure inflation.

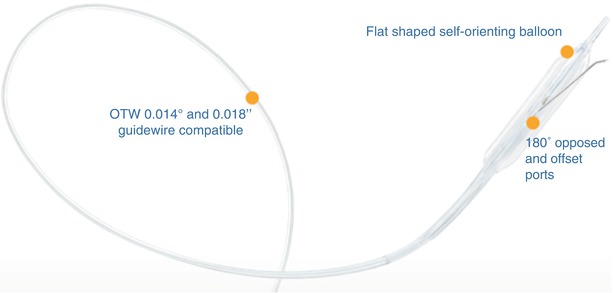

Fig. 31.6
Viance CTO Crossing Catheter (Covidien) – this catheter has a rounded tip which facilitates crossing via rapid rotation from external torque system

Fig. 31.7
Enteer balloon orientation catheter for lumen reentry – offset sideports consistently orient for true-lumen access (Courtesy of Covidien, Mansfield, MA)
The location of the true lumen is determined with fluoroscopic guidance. When the balloon is at the desired reentry location, the primary guidewire is removed, and the barbed reentry guidewire is introduced. The 0.014-in. reentry guidewire has an angled tip that is designed to be introduced through the appropriate exit port. Once the reentry wire is in the true lumen, the reentry balloon is removed followed by wire exchange and intervention.
Atherectomy Devices
PAD is a progressive chronic disease that requires a continuum of treatment, where few patients are treated once. Treatments that restore blood flow without reducing the ability to treat the vessel in the future are better suited for PAD. Atherectomy is seen by many as an ideal therapy for PAD because it can restore flow while removing the plaque burden and does not leave behind a permanent obstacle to future treatments such as a stent. There are currently four atherectomy systems approved in the United States.
Laser Atherectomy
Continuous-wave, hot-tipped lasers were evaluated and abandoned for peripheral arterial use in the late 1980s because of high complication rates caused by thermal damage to surrounding tissue [16]. In contrast, excimer laser-assisted angioplasty of the leg arteries has been practiced commercially in Europe since 1994. The Turbo Elite and Turbo-Tandem (Spectranetics) are commercially available excimer laser devices indicated for atherectomy of infrainguinal arteries (Fig. 31.8). These devices use flexible fiberoptic catheters to deliver intense bursts of ultraviolet energy in short pulse durations. The advantage of ultraviolet light lies in its short penetration depth and in its ability to break molecular bonds directly by a photochemical rather than thermal process. The excimer laser catheter removes a tissue layer of 10 μm with each pulse of energy. Tissue is ablated only on contact, without a consequent rise in temperature to surrounding tissue. Current data also suggests that ultraviolet light ablates thrombus and inhibits platelet aggregation [17].