Patient and Lesion Selection for Endovascular Management of Claudication
The therapeutic goal of endovascular treatment in patients with claudication is improving quality of life. Endovascular procedures are primarily indicated for claudicants who have an inadequate response to exercise or pharmacological therapy. Clinical outcomes are most durable for TASC type A iliac and femoropopliteal arterial lesions [6]. This is a class I indication in the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for the management of patients with peripheral arterial disease [6]. Note that the guideline-based support for endovascular intervention for the management of claudication is limited to suprapopliteal lesions – there is no routine indication for infrapopliteal percutaneous interventions for claudication.
Translesional pressure gradients (with and without vasodilation) should be obtained for the evaluation of lesions of intermediate (50–70 % diameter) angiographic severity. However, catheter-derived pressure gradient measurements across vascular lesions may lead to overestimation of the physiologic significance of these lesions and thus to inappropriate intervention. Instead, use of a pressure wire for hemodynamic interrogation may be a better tool for the assessment of the hemodynamic significance of a peripheral vascular lesion [7]. A study from our group reported the association between an invasive hemodynamic measure (translesional gradient), patient symptom status (assessed by a walking impairment questionnaire), and a noninvasive measure of ankle-brachial index in the infrainguinal arterial distribution. A translesional gradient ≥11 mmHg (assessed with a 0.014″ pressure wire) after 100 μg of intra-arterial adenosine was 100 % specific, reasonably sensitive (sensitivity 71.43 %), and provided the greatest discriminating ability to diagnose symptomatic PAD. Assessment of lower extremity hyperemic translesional gradients could have several important applications in daily clinical practice. It could allow the determination of the functional severity of arterial lesions in patients who undergo angiography for PAD symptoms, potentially target treatment of such lesions, and assess response to endovascular treatment [8].
There is ample evidence for the durability of stents as a primary therapy for common iliac and external iliac lesions, with particularly robust data for common iliac stenting. Stents may be provisionally used in all iliac artery distributions for suboptimal or failed balloon angioplasty (PTA). Failed iliac PTA is generally defined as a persistent translesional gradient, residual diameter stenosis greater than 50 %, or flow-limiting dissection [9].
The effectiveness of stents, atherectomy, cutting balloons, and lasers for the treatment of femoral-popliteal arterial lesions is not well established. In fact, there is a notable absence of guideline support, favorable case studies, or even single-arm registries. However, many of these devices have nonetheless become standard during endovascular interventions [6, 10].
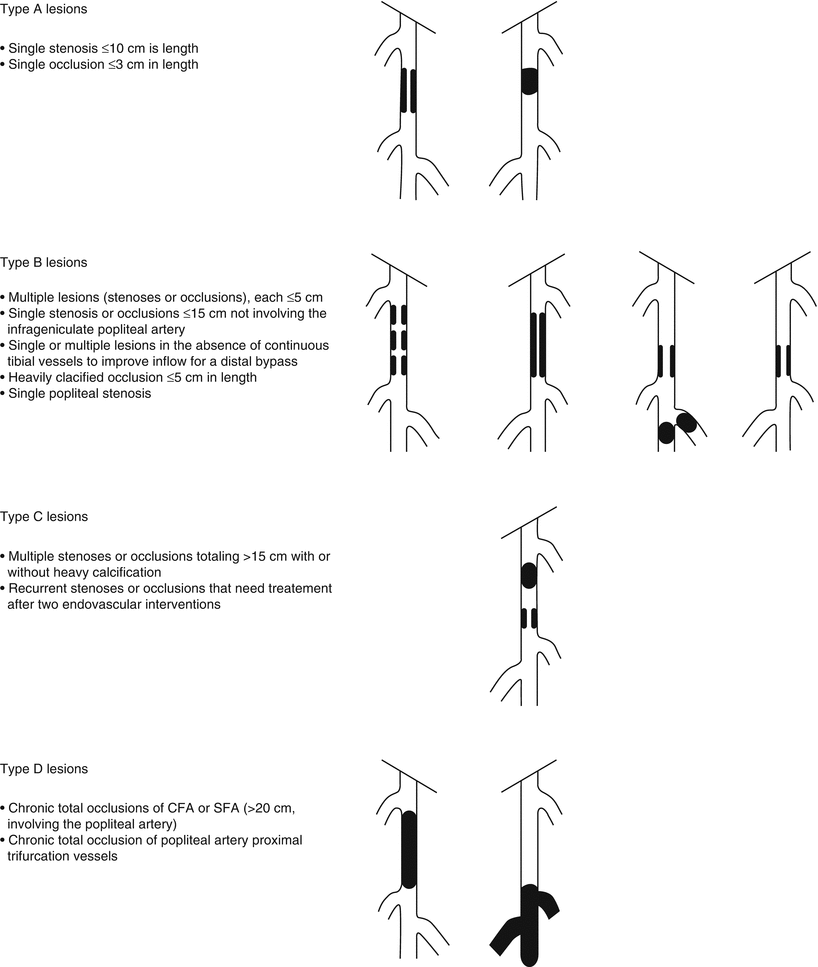
Finally, some lesions are best approached as a hybrid procedure with both endovascular and surgical therapies. Suprainguinal iliac artery atherosclerosis often involves the distal aorta and may extend across the inguinal ligament into the common femoral artery. Therefore, it is not uncommon to view iliac artery disease as an aortoiliac process. Figure 30.3 illustrates some common patterns of aortoiliac disease. Extension of this disease process below the inguinal ligament constitutes a major obstacle to endovascular therapy, and the presence of occlusive disease may complicate infra-inguinal surgical reconstruction, given the lack of suitable inflow. Given these challenges, some patients with aortoiliac occlusive disease with infrainguinal extension may be managed using hybrid procedures. An example of such a hybrid procedure would involve stenting of the iliac arteries into the common femoral artery, with direct repair of the common femoral by endarterectomy with or without path angioplasty or an interposition graft [11].
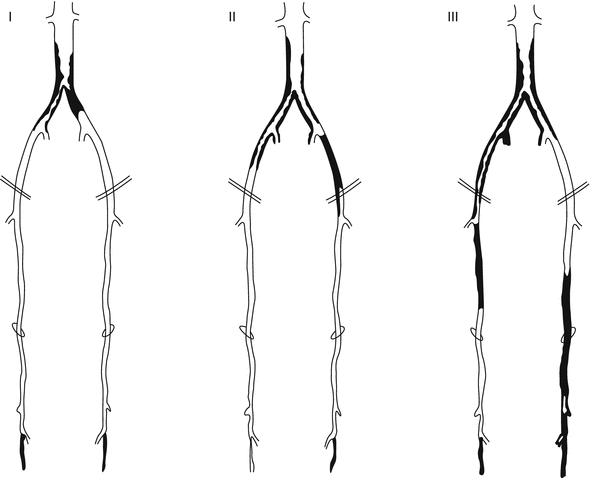

Fig. 30.3
Distribution of aortoiliac occlusive disease
Patient and Lesion Selection for Endovascular Management of Critical Limb Ischemia
Critical limb ischemia (CLI) is defined by the presence of rest pain, ulcers, or tissue loss attributed to arterial occlusive disease and is associated with great morbidity and mortality. Acute critical limb ischemia refers to patients in whom CLI symptoms have been present for <14 days, whereas symptoms persisting beyond 14 days are referred to as chronic critical limb ischemia.
Therapeutic goals of endovascular treatment of patients with CLI include relieving ischemic pain, healing ulcers, preventing major amputation, improving quality of life, and increasing survival [12]. Findings of the BASIL trial show that patients with severe limb ischemia, with a life expectancy of >2 years, and with a usable vein may derive greater benefit from bypass surgery because of the long-term patency of the saphenous vein. However, in the subset of patients with life expectancy of <2 years or those without an adequate vein, endovascular intervention should be considered as results of prosthetic bypass show poor durability in infrainguinal lesions [13]. It is important to note that given the diverse presentations and anatomic variations of PAD in patients with CLI, endovascular treatment should be undertaken exclusively by highly skilled and experienced physicians or groups of specialists.
In patients presenting with CLI with combined inflow (aortoiliac) and outflow (iliofemoral or femoropopliteal) disease, endovascular treatment should first be directed to inflow lesions [14]. However, restoration of pulsatile flow to the foot is generally needed for the healing of ischemic ulcers or gangrene. Therefore, if ischemic ulcers, gangrene, or infection persist after treatment of inflow disease and the ABI is below 0.8, an outflow vessel intervention should be performed [14].
Traditionally, procedural success of an endovascular intervention in CLI has been defined as <30 % residual diameter stenosis of critical inflow vessels and straight-line outflow in at least one infrapopliteal vessel to the pedal arch [15]. However, this definition fails to require targeted revascularization of ischemic tissue, and likely for this reason, nontargeted below-the-knee vessel interventions often lead to unsatisfactory clinical outcomes. Targeted therapy is possible using an angiosome-based approach, which provides a three-dimensional view of the tissue territories supplied by source arteries and has been used by general surgeons and plastic surgeons for limb and tissue salvage [16]. In a study comparing an angiosome-guided direct approach to CLI intervention compared to an indirect approach not angiosome guided, the limb salvage rate was significantly higher in the direct group than in the indirect group (86 % vs. 69 %, p = .03 at 1 year; 82 % vs. 64 %, p = .029 at 2 years; 82 % vs. 64 %, p = .029 at 3 years; and 82 % vs. 64 %, p = .029 at 4 years) [17].
Acute critical limb ischemia is a clinical emergency requiring rapid evaluation and treatment. Treatment begins with immediate anticoagulation with unfractionated heparin. Based on the results of several randomized trials, there is no clear superiority for endovascular management versus surgery on 30-day limb salvage or mortality, though surgery is used more often in practice [18]. Endovascular management options include catheter-based thrombolysis or thrombectomy [19].
Endovascular Intervention of Iliac Arteries
Surgical aortobifemoral bypass for iliac artery PAD has excellent 1-, 3-, and 5-year patency rates, and all iliac procedures, including endovascular interventions, are benchmarked against it. However, where outcomes between endovascular procedures and surgery are similar, endovascular procedures are favored due to the lower morbidity associated with minimally invasive approaches. The TASC guidelines specify that endovascular therapy is the treatment of choice for TASC A lesions and surgery the treatment of choice for TASC D lesions. They are less clear as to the optimal management of TASC B and C lesions, stating that in general endovascular treatment is preferred for type B lesions and surgery preferred for good-risk patients with type C lesions, but this is with the caveat that it should be an individualized choice for these lesions taking into account comorbidities and patient preferences [5]. It is worth noting that the guidelines might be lagging behind contemporary practice, with endovascular therapies increasing being chosen even in TASC type C and D lesions with good results.
Acute procedural success of PTA of iliac stenoses is >90 % overall. It is 100 % for focal iliac lesions and 80 % for long lesions and occlusions. While considering the management of iliac artery disease, it is critical to carefully evaluate the distribution and severity of atherosclerotic disease. Five-year patency rates of PTA for iliac lesions are between 70 and 72 % [20], and Murphy et al. reported an 8-year primary patency rate of 74 % with iliac artery stenting [21].
The Dutch Iliac Stent Trial compared routine iliac artery stenting to PTA with a provisional stent approach [22]. However, there was a high crossover rate with nearly half (43 %) of the patients randomized to PTA requiring stenting for a suboptimal result during the primary procedure. Re-intervention rates were similar in both arms at 2 (7 % PTA and 4 % stenting) [22] and 5 years (18 % PTA and 20 % stenting) [23]. There were no differences in iliac patency, ankle-brachial index (ABI), or quality of life between the two groups. A 2,116 patient meta-analysis comparing iliac artery PTA and stenting in claudicants and CLI reported a higher technical success rate with stenting; however, 30-day mortality was similar [9]. The 4-year primary patency rates were 65 % (PTA) and 77 % (stent) for stenoses and 54 % (PTA) versus 61 % (stent) for occlusion, with the inclusion of all reported technical failures.
Endovascular treatment of aortoiliac bifurcation lesions is not feasible with PTA but can be performed with high technical success with implantation of “kissing” stents, with 78 and 98 % 3-year primary and secondary patency rates [24]. High primary and secondary patency rates of 79 and 87 % at 1 year and 69 and 81 % at 3 years, respectively, have also been reported for stenting of iliac artery chronic total occlusions [25].
There are three types of stents typically used in peripheral interventions: uncovered balloon-expandable, covered balloon-expandable, and self-expanding stents. The type of stent chosen for a particular intervention is often determined by lesion location as well as lesion characteristics. There are no head-to-head studies of balloon-expandable versus self-expanding stents in the iliac distribution; however, there are studies comparing various covered versus bare-metal stent types, as discussed in more detail below.
Generally, in aortoiliac interventions, uncovered balloon-expandable stents are preferred over self-expanding stents. This is true for several reasons. Balloon-expandable stents have better radial strength, which is useful in heavily calcified lesions. Also, balloon-expandable stents have minimal foreshortening and allow for precise stent placement, which make them particularly suitable for stenting into the aorta using the kissing stent technique. The 2-year results of the MELODIE trial reported the safety and effectiveness of the balloon-expandable Express LD Vascular stent for the treatment of atherosclerotic iliac artery disease [26]. Primary patency was 92.1 % at 6 months and 87.8 % at 2 years. The rate of major adverse events (MAE) was 6.3 % at 6 months and 10.2 % at 2 years. Of the three event types included in the definition of MAE, only target lesion revascularization occurred, and 2-year ABI remained significantly improved compared to baseline (0.85 vs. 0.63, p < 0.0001). Most iliac stents studied are made up of stainless steel; however, the Assurant balloon-expandable cobalt stent has received Food and Drug Administration (FDA) approval for iliac artery implantation. The ACTIVE trial (unpublished; ClinicalTrials.gov Identifier: NCT00753337) reported a rate of 0.8 % for major adverse events, target lesion revascularization (TLR) and target vessel revascularization (TVR). In addition, the device also achieved a 99.2 % primary patency rate through 9 months of follow-up.
Covered balloon-mounted stents are generally reserved for complications or lesions with features which suggest high risk for embolization, i.e., eccentric ulcers or exophytic calcifications. A recent trial, COBEST (Covered Versus Balloon Expandable Stent Trial), of 125 patients with severe aortoiliac disease demonstrated that in the overall cohort, lesions treated with a covered stent were significantly more likely to remain free from binary restenosis than those that were treated with a bare-metal stent (hazard ratio [HR], 0.35; 95 % confidence interval (CI), 0.15–0.82; p = .02). Subgroup analyses demonstrated that covered and bare-metal stents produced similar and acceptable results for TASC B lesions; however, covered stents performed better for TASC C and D lesions in terms of long-term patency and clinical outcomes [27].
Self-expanding stents provide greater flexibility and are best suited for external iliac and more downstream lesions. A study comparing outcomes of two self-expanding stents for the treatment of iliac artery lesions reported 1-year primary patency rates of 94.7 and 91.1 % (not significant), respectively, with similar complication and symptomatic improvement rates regardless of the type of stent [28].
In conclusion, for type A iliac lesions, endovascular therapy is appropriate. For type D, surgery is most appropriate. For classes B and C, neither approach is clearly superior to the other, but endovascular therapy is more commonly used for class B and surgery more common for class C. Factors negatively affecting the patency of endovascular iliac interventions include poor quality of runoff vessels, severe limb ischemia, and long length of diseased segments. Female gender has also been suggested to decrease patency of external iliac artery stents [29].
Most iliac artery interventions are performed using common femoral access. During aortoiliac interventions careful analysis of the distal aorta and iliac vessel angiograms should be performed. Accessory renal arteries are found in approximately 15 % of patients and may arise from a variety of locations, such as the distal infrarenal abdominal aorta. Failure to recognize this variant during aortoiliac interventions could result in jailing and possibly occluding the renal artery, which could lead to ischemic renal injury. Using a wide (>12-in. diameter) image intensifier and digital subtraction angiography could aid in the recognition of this arterial variant. Transradial or brachial artery access for iliac interventions is feasible and is associated with lower periprocedural bleeding complications. These accesses are best suited for crossing flush occlusions of the proximal common iliac arterial segments, in patients with common femoral artery disease or patients with infrainguinal grafts [30]. Figure 30.4 demonstrates a case of successful endovascular treatment of a left common iliac artery through radial artery access.
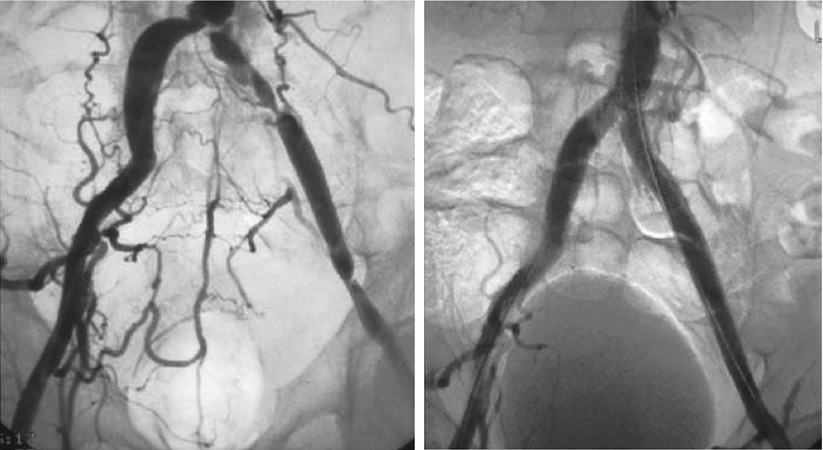

Fig. 30.4
Successful endovascular treatment of the left common iliac artery
Approximately one-third of obstructive lesions in peripheral arterial occlusive disease affect the aortoiliac segment. One of the main challenges when treating completely occluded iliac arteries is crossing the occluded segment safely. Retrograde, crossover, and brachial access individually or dual access is often required for endovascular treatment of iliac total occlusions. A primary guide wire (0.035″) generally with a support catheter can be used; however, blunt microdissection techniques (Frontrunner® and CrossBoss®) are becoming the more popular approach. Subintimal entry of the guide wire may occur during recanalization of an aortoiliac occlusion. Though experience with specific reentry devices in the iliac arteries is limited, the Pioneer system may be preferable because of its intravascular ultrasound-based orientation that may facilitate positioning of the device and reentry into the true lumen of the distal aorta [31].
Endovascular Intervention of Infrainguinal Vessels
The primary reason for the selection and ultimately exponential growth in infrainguinal endovascular procedures is to avoid the morbidity and mortality associated with open surgical reconstructions. Especially for lesions <10 mm in length, PTA of infrainguinal arterial vessels may be the preferred option [32]. The gap between the findings of the revised TASC document and contemporary endovascular practice worldwide is nowhere as wide as for the femoropopliteal and below-the-knee (BTK) interventions. This is primarily due to recent advancements in device development and advances in technical approach, all of which have increased success of endovascular treatment of complex femoropopliteal and chronic total occlusions to rates >85 %. The newer devices include hydrophilic guide wires, microdissection, and reentry catheters [32]. Therapeutic effectiveness of an endovascular approach to infrainguinal disease is dependent on the complexity of the underlying disease and clinical presentation of the patient. The immediate technical success and short- and intermediate-term patency rates achieved with the traditional endovascular modalities have been promising, with overall immediate technical and clinical success rate of PTA of femoropopliteal artery stenoses in all series exceeding 95 % [33]. However, long-term patency rates in the treatment of femoropopliteal disease have proved disappointing. The usefulness of conventional angioplasty is limited by elastic recoil, flow-limiting dissection, and high restenosis rates. The efficacy of stents has been limited by high restenosis and stent fracture rates.
In a balloon angioplasty performance analysis performed by Rocha-Singh et al., 12-month duplex ultrasound-derived patency was established to be low, at 33 % [34]. In a meta-analysis of balloon angioplasty and stent implantation studies for occlusive femoropopliteal PAD, Mwipatayi et al. reported a 45–84.2 % and a 25–77.2 % 1-year and 2-year primary patency rates following balloon angioplasty, compared to a 63–90 % 1-year and 46–87 % 2-year primary patency rates in the stent implantation group [27]. Based on these findings, the investigators concluded that stent placement for femoropopliteal occlusive disease does not increase the patency rate when compared with angioplasty alone at 1 year. Kasapis et al. in a meta-analysis of 10 randomized controlled trials compared routine stent implantation to PTA for femoropopliteal artery disease in 724 limbs treated with stents and in 718 limbs treated with balloon angioplasty and provisional stent strategy [35]. The follow-up period was 9–24 months, mean lesion length 45.8 mm in the stent and 43.3 mm in the provisional stent groups, respectively. Immediate technical failure was significantly higher in the balloon angioplasty with provisional stent group than in the stent group (17.1 % vs. 5.9 %, relative risk (RR) = 0.28, 95 % CI 0.15–0.54, p = 0.001). There was a trend for lower restenosis in the stent group, 37.6 % versus 45.3 % in provisional stent group (RR 0.85, 95 % CI 0.69–1.06, p = 0.146). There was no difference in the need for target vessel revascularization (20 % in-stent group vs. 20.2 % in provisional stent group, RR 0.98, 95 % CI 0.78–1.23, p = 0.89). These data do not support use of routine stenting as the primary endovascular treatment for short SFA lesions. A meta-regression analysis in the study from Kasapis et al. suggested that stenting may be beneficial for longer lesions [35, 36]. Despite methodological robustness, these findings were limited by the study’s inability to account for the wide variability of endovascular techniques and technologies used, limiting the practical relevance of such data. Data from more recent randomized clinical trials are also not conclusive. In the Femoral Artery Stenting Trial (FAST), Krankengerg et al. reported 1-year binary restenosis rates of 38.6 and 31.7 % with balloon angioplasty and nitinol stents implants in the SFA, respectively [36]. These findings are in contrast to another randomized study performed by Schillinger et al. who observed a 12-month binary restenosis rate of 36.7 % in their SFA nitinol stent group patients, compared to 63.5 % in the balloon angioplasty group. It is important to mention that the mean lesion length was approximately 45 mm in FAST and 101 mm in the study by Schillinger et al [37, 38]. Moreover, in FAST, significantly fewer chronic total occlusions of the SFA were included in the balloon angioplasty arm compared to the stent arm, and stenting with nitinol self-expanding stents showed a 15 % lower restenosis rate in diabetic subjects. The findings from the Randomized Study Comparing the Edwards Self-Expanding Lifestent versus Angioplasty-alone In Lesions Involving the SFA and/or Proximal Popliteal Artery (RESILIENT) trial also show that angioplasty is effective for certain types of lesions, particularly those under 5 cm in length [38].
The subset of PAD patients with diabetes deserves special attention. Patients with diabetes have significantly more diffuse and angiographically more severe PAD. An observational study of 65 consecutive patients who underwent long-segment (≥10 cm) SFA stenting found that 40 % of patients developed in-stent restenosis during the median 8-month follow-up. Cumulative freedom from restenosis at 6 and 12 months was 79 and 54 %, respectively, for the overall cohort. However, in diabetics, the 6- and 12-month freedom from restenosis was 68 and 22 %, respectively, compared to nondiabetics, with rates of 84 and 71 %, at the same time periods (adjusted HR of 3.8, p = 0.01) [39]. This observation of high restenosis rates in patients with diabetes mellitus, especially after long-segment femoropopliteal stenting using self-expanding nitinol stents, has been confirmed by other studies and remains a challenging clinical problem. Despite these observations, SFA stenting remains highly prevalent in patients with diabetes mellitus, given the dismal (<30 %) 1-year patency rates following balloon angioplasty of long segments of stenosed or occluded SFAs.
Endovascular Intervention of Popliteal and Infrapopliteal Vessels
The management of popliteal and infrapopliteal arterial disease is challenging, and surgery remains the gold standard for treating infrapopliteal disease. The majority of patients with symptomatic PAD of the popliteal and infrapopliteal arteries have multilevel disease with atherosclerosis in the aortoiliac and femoropopliteal segments as well, with a minority of patients with symptomatic disease from isolated popliteal or infrapopliteal PAD [5, 40]. The anatomic challenges of intervening on the small and often heavily calcified tibial and pedal arteries have led to the generally inferior results of infrapopliteal revascularization compared to revascularization of larger proximal arterial segments, and consequently, intervention in patients with infrapopliteal PAD is generally reserved for patients with CLI, as these patients have the highest risk of limb loss and death [41].
If endovascular treatment of an infrapopliteal lesion is ultimately decided upon, evaluation and treatment of proximal lesions (aortoiliac or femoropopliteal) is usually necessary for successful treatment of lesions at or below the knee [42




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree