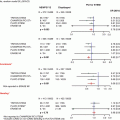
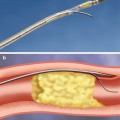
Fig. 35.1
Assessment of severity of carotid stenosis. The NASCET uses the formula b − a/b × 100, where a is the residual luminal diameter at the stenosis and b is the luminal diameter at a visible, disease-free point above the stenosis. The ECST uses the formula c − a/c × 100, where c is the estimated luminal diameter at the level of the lesion based on a visual impression of where the normal arterial wall was before development of the stenosis
Diagnosis of Carotid Disease
Indications for Screening
In asymptomatic patients, there are no guidelines to support routine screening for atherosclerotic carotid artery disease. In patients with carotid bruits discovered on routine examination, further diagnostic tests for identification of carotid disease are recommended if they are candidates for carotid revascularization [1]. In patients scheduled for coronary artery bypass graft surgery (CABG), screening for carotid stenosis is recommended in patients older than 65 years, those with left main coronary stenosis, known peripheral arterial disease, a history of smoking, a history of TIA or stroke, or in the presence of a carotid bruit [32]. Patients with transient retinal or hemispheric neurological deficits should be screened for extracranial carotid artery disease [1, 32].
Diagnostic Modalities
Current management decisions for atherosclerotic carotid disease depend heavily on the severity of luminal stenosis. Therefore, accurate grading of stenosis severity is essential to good decision-making.
Noninvasive Testing
The three noninvasive modalities used for diagnosis of carotid disease are Doppler ultrasound (DUS), magnetic resonance angiography (MRA), and computerized tomographic angiography (CTA). Among the noninvasive imaging techniques, DUS is the most widely available, least toxic, and least expensive. It has been established as an accurate method of assessing carotid stenosis and provides reliable information on the localization and degree of stenosis, plaque structure, and vessel wall characteristics [33, 34]. Diagnostic accuracy of DUS is limited by operator skill and has susceptibility to artifacts from calcified plaques [35]. In addition, it does not provide an overview of vascular anatomy and surrounding structures [36]. Although consensus criteria for interpreting carotid duplex ultrasound results have been published [37], it is important to note that data obtained by individual vascular laboratories will vary owing to differences in equipment, ability, and consistency of vascular technicians and reader interpretability. Therefore, each vascular laboratory needs to internally validate their criteria for definition of carotid stenosis [38]. A meta-analysis of 64 series comparing DUS with digital subtraction angiography (DSA) found a sensitivity of 86 % and a specificity of 87 % for detecting stenosis of 70–99 % by NASCET criteria [39].
MRA can quantify carotid stenosis noninvasively and does not expose the patient to ionizing radiation or ionizing contrast. There is concern about administering gadolinium to patients with renal insufficiency. MRA offers 3D reconstructions of the carotid artery and the possibility of “one-stop” brain imaging. MRA, however, tends to overestimate the degree of stenosis [39]. A meta-analysis of 17 studies looking at 1,714 carotids in 905 patients comparing contrast-enhanced MRA (CE-MRA) with DSA found a sensitivity of 94 % and a specificity of 92 % for identification of carotid stenosis of 70–99 % by NASCET criteria [40].
CTA exposes the patient to both ionizing radiation and iodinated contrast, but has several advantages over DUS and MRA. CTA has a better spatial resolution than MRA, is less susceptible to artifacts, and details vascular anatomy. A meta-analysis comparing CTA with DSA found sensitivity ranging from 85 to 92 % and specificity ranging from 93 to 97 % for detecting stenosis of 70–99 % by NASCET criteria [41, 42].
Invasive Testing
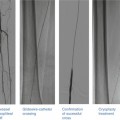
DSA is considered the gold standard in terms of assessment of luminal stenosis. Cerebral catheter-based angiography carries a risk of cerebral infarction of 0.5–1.2 %; therefore, noninvasive imaging should be the initial strategy for evaluation [19, 43]. Vascular access is most commonly obtained from the femoral artery although brachial or radial access may be used (Fig. 35.2). Prior to selective arch angiography, an arch aortogram is performed with a pigtail catheter placed in the proximal ascending aorta to define the anatomy of the aortic arch. This is done in the 45° left anterior oblique (LAO) position with a large-format image intensifier (12-in. to 16-in.) using DSA (15 cm3 per second for 3 s) and a power injector. Once the morphology of the aortic arch is determined (Fig. 35.3a), catheters are chosen for selective angiography of the cervical arteries supplying the brain (right and left carotid and vertebral arteries) and the cerebral vasculature (Fig. 35.3b). In a type I arch, Berenstein or Judkins Right (JR) catheters are often used. In type II or III arch morphologies, shepherd’s crook-shaped catheters, i.e., Simmons or Vitek catheters, may be best (Fig. 35.4).
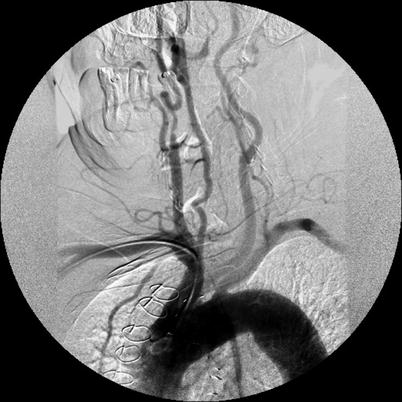
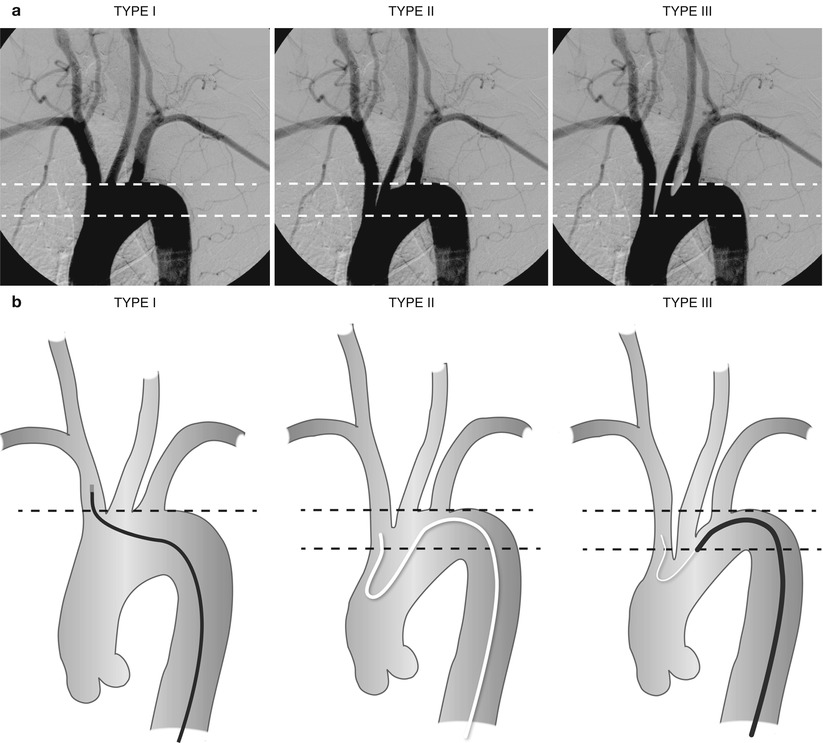
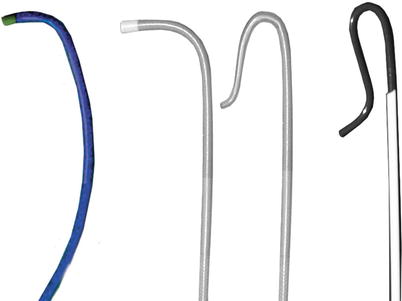

Fig. 35.2
Radial access for aortic arch angiography

Fig. 35.3
(a) Aortic arch morphologies. Type I arch: all vessels originate at the superior margin of the arch. Type II arch: at least 1 vessel originates between the superior and inferior margins of the aortic arch. Type III arch: at least 1 vessel originates below the inferior margin of the aortic arch. (b) Aortic arch morphologies with different catheters used to access the right innominate artery. A JR 4 catheter is used in a type 1 arch, Simmons catheter for a type 2 arch, and Vitek catheter for a type 3 arch

Fig. 35.4
Catheters for accessing the carotid artery. Berenstein or JR 4 catheters are usually best for a type 1 arch. Simmons and Vitek catheter may be best for type 2 or type 3 arches
Angiograms are obtained to delineate the anterior and posterior circulation supplying the brain. The intracranial and extracranial portions of each vessel are studied. We routinely obtain three views of carotid bifurcation (anterior, lateral, and a 45° oblique views). Alternatively, some operators use rotational angiography imaging a 90° arc with a single injection. It is important to demonstrate the anatomy of the Circle of Willis to define any baseline abnormalities. DSA may be performed with diluted contrast, using a 1:1 mix of saline and contrast. A reference object is used with carotid angiograms in order to accurately measure the diameter of the artery. The NASCET criteria for measuring carotid stenosis have become the international standard methodology (Fig. 35.1).
Medical Therapy
Atherosclerotic risk factor management is recommended for patients with documented atherosclerotic carotid stenosis similar to that for patients with coronary artery disease. This includes blood pressure (BP) control, glycemic control in diabetics, statins for management of dyslipidemia, and cessation of smoking and heavy alcohol consumption [44]. The optimal antihypertensive drug regimen remains uncertain. The recently published extracranial carotid and vertebral artery disease guidelines have recommended a BP goal of less than 140/90 [45]. In patients with symptomatic carotid stenosis, a goal low-density cholesterol (LDL) level of <70 mg/dl or a reduction of at least 50 % in LDL is recommended [44, 46]. In patients with an ischemic stroke or TIA, aspirin monotherapy (50–325 mg/day), aspirin 25 mg and dipyridamole 200 mg twice daily, or aspirin 25 mg and clopidogrel 75 mg daily are all acceptable options for initial therapy. In patients presenting with an ischemic stroke on daily aspirin, there is no benefit for increasing the daily dose of aspirin or adding another antiplatelet agent (clopidogrel or dipyridamole). In patients with underlying symptomatic carotid dissection, antithrombotic treatment (unfractionated heparin, low molecular weight heparin, or Coumadin) for 3–6 months is reasonable [44].
Clinical Trials
Large-scale randomized trials (RTs) have been conducted to compare medical treatment with surgical revascularization in patients with both asymptomatic and symptomatic atherosclerotic carotid artery stenoses. There is current debate whether more aggressive modern anti-atherosclerotic therapy would reduce the magnitude of benefit shown for surgery in the earlier trials.
In the last decade, percutaneous treatment of carotid stenosis with protected carotid artery stenting (CAS) has emerged as an attractive alternative to carotid endarterectomy (CEA). Data on over 24,000 patients undergoing CAS have been published. In addition, more than 12 RTs and several industry-sponsored CAS registries have described outcomes of CAS in patients for patients at average and high risk for CEA [47]. Patients at increased risk for CEA are defined by anatomic characteristics and medical comorbidities as outlined in Table 35.1.
Table 35.1
High-risk criteria for carotid endarterectomy per clinical expert consensus document 2007
Anatomic criteria | Medical comorbidities |
|---|---|
Lesion at C-2 or higher | Age ≥ 80 |
Lesion below clavicle | NYHA class 111/1V congestive heart failure |
Prior radical neck surgery or irradiation | CCS class 111/1V angina |
Contralateral carotid occlusion | Left main/≥2 vessel coronary artery disease |
Prior ipsilateral carotid endarterectomy | Urgent <30 days heart surgery |
Contralateral laryngeal nerve palsy | Left ventricular ejection fraction ≤30 % |
Tracheostoma | Recent (<30 days) myocardial infarction |
Severe chronic lung disease | |
Severe renal disease |
Medical Therapy Versus CEA in Asymptomatic Patients
Three randomized trials compared carotid endarterectomy (CEA) plus aspirin with aspirin alone in asymptomatic patients. In the VACS trial, 444 men with asymptomatic stenosis of ≥50 % by angiography (NASCET criteria) were randomized to medical therapy plus CEA or medical therapy alone [48]. There was no difference in ipsilateral stroke (CEA 4.7 % vs. medical 9.4 %, P = NS) or combined end point of stroke and death (CEA 41.2 % vs. medical 44.2 % P = NS) at a mean follow-up of 4 years.
The ACAS trial randomized 1,662 patients with asymptomatic carotid stenosis of ≥60 % by angiography (NASCET criteria) to medical therapy plus CEA or medical therapy alone [19]. The estimated 5-year risk of ipsilateral stroke and any perioperative stroke or death was 11.0 % for the medical group and 5.1 % for the surgical group (relative risk reduction 53 %, 95 % CI, 22–72 %, P = 0.004). The risk of stroke and death for patients undergoing CEA was the highest during the first month after endarterectomy, whereas the risk for medical patients was evenly distributed throughout 5 years. Using ACAS eligibility requirements, 19 surgeries would be necessary to prevent one stroke over 5 years. Although not statistically significant, the benefit of CEA was greater in men when compared to women (reduction of 5-year event rate; men 66 %, 95 % CI, 36–82 % vs. women 17 %, 95 % CI, –96–65 %, P = 0.10). There was no relationship for risk of stroke and increasing percent stenosis in the medical treatment group. A post hoc analysis suggested that CEA in patients with contralateral occlusion provides no long-term benefit and may be harmful [49].
ACST evaluated 3,120 asymptomatic patients with ≥60 % carotid stenosis by ultrasound [23]. The risk of perioperative stroke or death was 3.1 % (95 % CI 2.3–4.1 %). The 5-year risk of perioperative death or total stroke was reduced from 11.8 to 6.4 % with CEA. The benefit of surgery was significant for both men and women and across varying degrees of stenosis (60–99 % stenosis). The 10-year risk of perioperative stroke or death was reduced from 17.9 to 13.4 % with CEA [50]. Half of this reduction was in disabling or fatal strokes.
Medical Therapy Versus CEA in Symptomatic Patients
Three large studies have been performed evaluating the benefit of CEA versus medical therapy in symptomatic patients with carotid artery disease. In these trials, medical therapy included smoking cessation, antiplatelet, antihypertensive, and lipid-lowering agents. However, similar to trials in patients with asymptomatic carotid stenosis, the end points of medical treatment were not specified, and the nature of the medical treatment varied with the time of patient enrollment and randomization [28, 29].
The VA 309 trial screened 5,000 men who presented within 4 months with a small stroke, TIA, or transient monocular blindness and randomized 189 to either CEA with best medical therapy or best medical therapy alone [51]. These patients had angiographically defined internal carotid stenosis >50 %. This trial was ended prematurely when early results from NASCET and ECST confirmed the significant benefit of CEA. At a mean follow-up of 1 year, there was a reduction in ipsilateral stroke or TIA from 19.4 % in the medical treatment arm to 7.7 % in the surgical arm (P = 0.011).
The NASCET trial investigators randomized 2,885 patients with a TIA or non-disabling stroke within 180 days of enrollment to CEA with medical therapy or medical therapy alone. Patients were originally stratified according to the degree of stenosis: <50, 50–69, and 70–99 %. After randomizing 659 patients with ≥70 % stenosis, that portion of the study was concluded. CEA demonstrated an absolute risk reduction of 17 % (26 % vs. 9 %, P < 0.001) in the rate of ipsilateral stroke at 2 years [6, 52]. Among patients with a 50–69 % stenosis, there was an absolute risk reduction of 6.5 % in the rate of ipsilateral stroke in those treated with CEA (22.7 % vs. 15.7 %, P = 0.04) after 5-year follow-up [28]. In patients with <50 % stenosis, there was no benefit for CEA compared to medical therapy. The 5-year rate of ipsilateral stroke was 18.7 % in those treated medically and 14.9 % in those treated with CEA (P = 0.16) [28]. For patients with stenosis >70 %, the perioperative risk of stroke or death was 5.8 %, whereas for those with stenosis 50–69 %, it was 6.7 %. The number needed to treat to prevent 1 stroke in a 2-year period was 8 for patients with stenosis >70 % and 20 for patients with stenosis 50–69 %.
The ESCT trial investigators randomized 3,024 patients with a TIA or non-disabling stroke within 180 days of enrollment to CEA with medical therapy or medical therapy alone [29]. In patients with ≥80 % stenosis by ESCT criteria (70 % stenosis by NASCET criteria), the rate of major stroke or death at 3 years was 26.5 % in the medical therapy group and 14.9 % in the CEA group, an absolute reduction of 11.6 % favoring surgery (P < 0.001). There was no benefit for patients who had near occlusion of the carotid artery. The perioperative risk of stroke or death was 7.0 %.
Current Recommendations for Carotid Endarterectomy
Based on the above data, CEA has been established as the preferred treatment to prevent stroke for average surgical risk symptomatic patients with ≥50 % carotid stenosis [28, 29] and average-risk asymptomatic patients with ≥60 % carotid stenosis [19, 23, 48] compared with medical therapy provided the perioperative risk of stroke and death do not exceed 3 % for asymptomatic patients and 6 % for symptomatic patients. Symptomatic patients should undergo CEA within 14 days of presenting event provided there are no contraindications for revascularization. Asymptomatic patients undergoing CEA should have a life expectancy of more than 5 years [44].
CEA Versus CAS in High-Risk Patients
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) was a multicenter, prospective RT that compared CAS with an embolic protection device (EPD) to CEA in patients at increased risk for surgery [53]. To be included, patients had to have symptomatic stenosis ≥50 % by angiography or asymptomatic stenosis ≥80 % by ultrasound with one or more high-risk features (Table 35.1) and be eligible for either CEA or CAS. Investigators in this trial had a median experience of 64 CAS procedures (range 20–700). Of 747 patients randomized, 334 were eligible for either CAS or CEA and entered the trial, and 413 patients were entered into a registry. Among 334 patients randomized, 167 underwent CEA and CAS, respectively. The primary end point of the trial was the cumulative incidence of death, stroke, or myocardial infarction within 30 days after the procedure or death or ipsilateral stroke between 31 days and 1 year. The 30-day death or stroke rate was 5.6 % for CEA and 4.8 % for CAS. Rates of 30-day MI were higher, although statistically nonsignificant, for CEA when compared to CAS (6.1 % CEA, 2.4 % CAS, P = 0.10). At 1 year, the primary composite end point was reached in 20.1 % of patients with CEA as compared to 12.2 % patients with CAS (P = 0.048 for non-inferiority). In addition, target vessel revascularization at 1 year (CEA 4.3 %, CAS 0.6 %, P = 0.04) and cranial nerve palsy rates (CEA 4.9 %, CAS 0 %, P = 0.03) favored stenting. On the basis of this data, in April 2004, an FDA panel advisory recommended approval of the devices used in this trial. Three-year follow-up of the SAPPHIRE trial continues to show no differences for the primary end point between CEA and CAS (CEA 30.3 %, CAS 24.6 %, P = 0.27) [54].
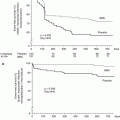
Following FDA approval of carotid stent systems for symptomatic high surgical risk patients with ≥70 % carotid stenosis, several post-market surveillance registries have been launched. An overview of RTs and registries for patients undergoing CAS is provided in Table 35.2 and Fig. 35.5.
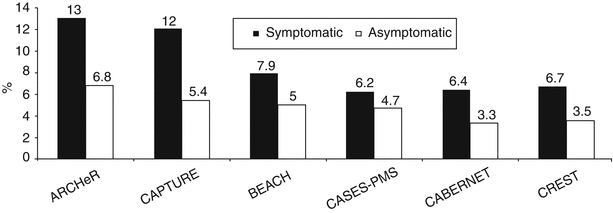
Table 35.2
Randomized trials of Carotid Artery Stenting
Variable | SAPPHIRE¶ | CREST | ICSS | EVA-3S | SPACE | Kentucky | KENTUCKY | CAVATAS | LEICESTER | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
2001 | Wallstent | BACASS | 2004 | ||||||||||
N = 334 | N = 527 | N = 1183 | N = 104 | N = 219 | N = 20 | N = 85 | N = 504 | N = 17 | |||||
Inclusion Criteria | High-risk CEA with symptomatic carotid stenosis ≥50 % or asymptomatic carotid stenosis ≥80 % | Symptomatic carotid stenosis ≥50 % | Symptomatic carotid stenosis >50 % | Symptomatic carotid stenosis ≥60 % | Symptomatic carotid stenosis ≥50 % | Symptomatic carotid stenosis >70 % | Symptomatic carotid stenosis >60 % | Symptomatic carotid stenosis ≥70 % | Asymptomatic carotid stenosis >80 % | Symptomatic Carotid stenosis | Symptomatic Carotid stenosis >70 % | ||
Asymptomatic carotid stenosis ≥60 % | |||||||||||||
Stent system | PRECISE | Acculink Carotid Stent | Wallstent, Precise, Acculink Smart Exponent | Wallstent, Precise, Acculink Zilver | Wallstent, Precise, Acculink | Wallstent | Wallstent | Wallstent | Wallstent | Wallstent, Stricker Palmaz (26 %) | Wallstent | ||
EPD | Angioguard XP | Accunet Embolic Protection Device | Percusurge, Angioguard, Filterwire EZ, Mednova, parody, Emboshield, etc. (operator discretion) | Percusurge Guardwire, Angioguard, Filterwire, EZ, Spider, Emboshield, Accunet | Percusurge, FilterWire EX, Neuroshield, carotid trap (in 27 %) | None | None | FilterWire, Angioguard RX | None | None | None | ||
Termination | Premature (%) | Trial completion (%) | Interim analysis (%) | Premature (%) | Premature (%) | Trial completion (%) | Premature (%) | Premature (%) | Trial completion (%) | Exploratory trial (%) | Premature (%) | ||
30-day death | 2.5, 1.2 | 0.3, 0.7 | 0.1, 0.1 | 1.2, 0.8 | 0.9, 0.7 | 2, 0 | NA | 0, 0 | 0, 0 | 2, 3 | 0, 0 | ||
CEA, CAS | |||||||||||||
30-day stroke | 3.1, 3.6 | 2.3, 4.1 | 3.2, 7.0 | 2.7, 8.8 | 6.2, 7.5 | 0, 0 | NA | 10, 0 | 0, 0 | 8, 8 | 0, 71 | ||
CEA, CAS | |||||||||||||
30-day MI | 6.1, 2.4 | 2.3, 1.1 | 0.6, 0.3 | 0.8, 0.4 | 0, 0 | 0, 0 | NA | 0, 0 | 0, 0 | NA | NA | ||
CEA, CAS | |||||||||||||
30-day death/stroke | 8.4, 5.5 | 2.3, 4.4 | 3.4, 7.1 | 3.8, 9.4 | 6.5, 7.7 | 2, 0 | 4.5, 12.1 | 10, 0 | 0, 0 | 10, 10 | 0,71 | ||
CEA, CAS | |||||||||||||
30-day death/stroke/MI | 9.8, 4.8 | 6.8, 7.2 | 4.0, 7.4 | 6.1, 11.7 | 6.5, 7.7 | 2, 0 | NA | 10, 0 | 0, 0 | NA | NA | ||
CEA, CAS |

Fig. 35.5
30-day rates of death, stroke, and myocardial infarction for carotid artery stenting
CEA Versus CAS in Average-Risk Patients
The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) trial, published in 2001, was the first multicenter clinical trial which randomized 504 symptomatic patients at average risk for CEA in 22 centers to CEA (253 patients) or endovascular revascularization (251 patients) [55]. In patients with technically successful endovascular revascularization, stents were used in only 26 % (55/213). The 30-day outcome of death and stroke was equivalent for both CEA and CAS (10 %). CEA had a higher rate of cranial nerve palsy and hematoma as compared to CAS (cranial nerve palsy 9 % CEA vs. 0 % CAS, P < 0.0001; hematoma 7 % CEA vs. 1 % CAS, P < 0.0015). The 1-year death or stroke rate was comparable, 13.4 % CEA versus 14.3 % CAS, P = NS. The 1-year restenosis rate (>70–99 %) was 14 % for CAS as compared to 4 % for CEA (P < 0.001). At 3-year follow-up, no difference in the rate of death or stroke was noted (14.2 % CEA vs. 14.3 % CAS, P = NS) [56].
Several multicenter randomized trials (RTs) comparing CAS to CEA for average surgical risk patients with both symptomatic and asymptomatic carotid stenoses have been published, while others are ongoing (Table 35.2). The Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial was designed to prove non-inferiority of CAS as compared to CEA in average surgical risk symptomatic patients with ≥50 % carotid stenosis by angiography [57]. It was a multinational, multicenter RT with the primary end point of ipsilateral stroke or death between randomization and 30-day follow-up. Initially, 1,200 patients were randomized between March 2001 and Feb 2006 (595 patients to CEA and 605 patients to CAS). Tutoring of inexperienced interventionalists enrolling study patients was permitted. Surgeons had to provide proof of 25 consecutive carotid endarterectomy procedures. At the time of an interim analysis, the primary end point occurred in 6.4 % of patients randomized to CEA and 6.8 % randomized to CAS (P = NS). The incidence of any stroke was 6.2 % in CEA and 7.5 % in CAS (P = NS). Importantly, EPDs were used in only 27 % of cases. Although no difference was seen between CAS and CEA, this analysis showed that 2,500 patients would be needed to prove non-inferiority of CAS. The SPACE steering committee acknowledged a lack of funds sufficient to enroll the requisite number of patients and suspended the trial.
The Endarterectomy Versus Angioplasty in patients with Symptomatic Severe carotid Stenosis (EVA-3S) trial was similar to the SPACE trial in that it was a RT designed to assess non-inferiority of CAS as compared to CEA in average-risk symptomatic patients with carotid stenosis [58]. The symptomatic carotid stenosis threshold was increased to >60 %. This was a multicenter trial conducted in France from 2000 to 2005. The primary end point was the incidence of 30-day death or any stroke. The trial was stopped after enrolling 527 of the intended 872 patients for reasons of safety and futility. Two hundred and sixty-two patients underwent CEA and 265 received CAS. The primary end point was lower in patients undergoing CEA as compared to CAS (3.8 % CEA vs. 9.4 % CAS, P = 0.01), and EVA-3S concluded that CAS was inferior to CEA for symptomatic patients with carotid stenosis >60 %. Critics of this study point to the fact that trial operators certified to perform CAS could have little (5 prior cases) or no experience (interventions performed with a tutor), and EPDs were used in a minority of the trial, after 5 out of 20 patients treated without EPD suffered a stroke. This resulted in protocol changes by the EVA-3S safety committee. At 4-year follow-up, excluding periprocedural complications, rates of death or any stroke were comparable between CEA and CAS (HR 1.39, 95% CI 0.96–2.00; P = 0.08) [58].
The International Carotid Stenting Study (ICSS) was another European RT that enrolled 1,713 (CAS = 855 or CEA = 858) symptomatic patients at average risk for CEA with carotid stenosis >50 % by angiography (NASCET criteria) [59]. Amazingly for a modern trial, the use of EPDs was optional. To qualify as an experienced center, a surgeon must have performed 50 CEA procedures and an interventionalist (physician or surgeon), 10 CAS procedures. However, if the center had no CAS experience, they could still enroll patients into the trial if they were tutored by a proctor.
The primary outcome of ICSS was the 3-year rate of any fatal or disabling stroke. The 3-month interim analysis demonstrated a similar result between the two groups (4.0 % for CAS and 3.2 % for CEA, P = 0.34). The 120-day rate of death, stroke, or MI was 8.5 % in the CAS group and 5.2 % in the CEA group (P = 0.006).
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) is the largest randomized trial published comparing CAS with EPD to CEA [60]. There were 2,502 average surgical risk patients with symptomatic (1,321) and asymptomatic (1,181) carotid stenosis randomized to either CEA or CAS. The primary outcome of periprocedural stroke, death, or MI or follow-up ipsilateral stroke was not different between the two groups at 4 years (7.2 % for CAS and 6.8 % for CEA). The 30-day risk of all stroke was higher for CAS (4.1 % vs. 2.3 %, P = 0.01), whereas CEA was associated with a higher 30-day risk of MI (2.3 % vs. 1.1 %, P = 0.03). The rate of ipsilateral stroke over a mean follow-up of 2.5 years was similar between the two groups. CAS trended safer than CEA for patients ≤69 years of age, while CEA yielded better outcomes in those >70 years of age.
The Asymptomatic Carotid Stenosis Stenting versus Endarterectomy (ACT I) trial will further help evaluate risk of CAS versus CEA in asymptomatic patients at average risk for CEA. This trial is enrolling 1,540 patients with asymptomatic stenosis greater than 70 % at average risk for CEA and randomizing them, on a 3:1 basis, to CAS or CEA [35]. The aim is to demonstrate the non-inferiority of CAS using the Emboshield Embolic Protection System and Emboshield Pro Embolic Protection System with the Xact Carotid Stent System (Abbott Vascular Corporation, Abbott Park, Illinois) to CEA for the treatment of asymptomatic extracranial carotid atherosclerotic disease. The primary end point is death, stroke, or MI at 30 days and ipsilateral stroke between 31 and 365 days. Secondary end points include target lesion revascularization, device and procedural success, ipsilateral stroke at 5 years, and economic and quality of life indicators.
Guidelines for Carotid Artery Stenting
The American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions/Society for Vascular Medicine and Biology/Society of Interventional Radiology/American Society of Interventional and Therapeutic Neuroradiology (ACCF/SCAI/SVMB/SIR/ASITN) published a clinical expert consensus document on carotid stenting in 2007 [1]. Per this consensus statement, CAS is recommended in patients with symptomatic stenosis greater than 50 % and asymptomatic stenosis greater than 80 % (NASCET criteria) who are at high risk for CEA.
For average surgical risk patients, the recently published American Heart Association stroke guidelines make CAS a level 1 indication for symptomatic patients with carotid stenosis >50 % by angiography at average risk for CEA. In patients at high-risk for CEA, CAS is indicated among patients with symptomatic stenosis >70 % provided perioperative risk morbidity and mortality rates are 4–6 %, respectively [44]. This document did not comment on revascularization for patients with asymptomatic carotid stenosis. The recently published guidelines on extracranial carotid and vertebral artery disease make CAS a class 1 indication for symptomatic patients with carotid stenosis >50 % by angiography (NASCET method) provided the anticipated rate of stroke or death is less than 6 %. For asymptomatic patients with carotid stenosis >60 % by angiography (NASCET method), CAS can be considered in highly selected patients [45]. A summary of recommendations for CAS by various professional societies is provided in Table 35.3.
Table 35.3
Recommendation for carotid artery stenting by professional societies
Professional society | Asymptomatic CAS | Symptomatic CAS |
|---|---|---|
ASITN/ASNR/SIR guideline 2003 | ≥90 % with high-risk features £ | ≥70 % with high-risk features £ |
SVS guideline 2008 | Not recommended | ≥50 % with high-risk features £ |
ESVS guideline 2009 | In high-volume centers with documented low periprocedural death or stroke rates (≤3 %) or within well-conducted clinical trials | In high-volume centers with documented low periprocedural death or stroke rates (≤6 %) or within well-conducted clinical trials or when anatomy is not amenable for CEA |
Stenosis threshold are not validated | Stenosis threshold are not validated | |
SPREAD Italian guideline 2009 | In patients with difficult to access anatomy for CEA or medical comorbidities | In patients with difficult to access anatomy for CEA or medical comorbidities |
Stenosis threshold are not mentioned | Stenosis threshold are not mentioned | |
ACCF/SCAI/SVMB/SIR/ASITN Consensus statement 2007 | >70 % with high-risk features £ | >50 % with high-risk features £ |
AHA stroke guidelines 2010 | >70 % with high-risk £ features/>50 % at average risk for CEA | NA |
ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS extracranial carotid and vertebral artery disease guidelines 2011 | >60 % by angiography |