Current indication
Class
Level of evidence
Symptomatic patients (NYHA functional class II, III, or IV), moderate or severe mitral stenosis (area <1.5 cm2), and valve morphology favorable for percutaneous balloon valvuloplasty in the absence of left atrial thrombus or moderate to severe mitral regurgitation
I
Grade A
Asymptomatic patients with moderate or severe mitral stenosis (area <1.5 cm2) and valve morphology favorable for percutaneous balloon valvuloplasty who have pulmonary hypertension (pulmonary artery systolic pressure >50 mmHg at rest or 60 mmHg with exercise) in the absence of left atrial thrombus or moderate to severe mitral regurgitation
IIa
Grade C
Patients with NYHA functional classes III–IV, moderate or severe mitral stenosis (area <1.5 cm2), and a nonpliable calcified valve who are at high risk for surgery in the absence of left atrial thrombus or moderate to severe mitral regurgitation
IIa
Grade B
Asymptomatic patients, moderate or severe mitral stenosis (area <1.5 cm2), and valve morphology favorable for percutaneous balloon valvuloplasty who have new onset of atrial fibrillation in the absence of left atrial thrombus or moderate to severe mitral regurgitation
IIb
Grade B
Patients in NYHA functional classes III–IV, moderate or severe mitral stenosis (area <1.5 cm2), and a nonpliable calcified valve who are low-risk candidates for surgery
IIb
Grade C
Patients with mild mitral stenosis
III
Grade C
Percutaneous mitral valvuloplasty (PMV) success depends on appropriate patient selection. A multifactorial score derived from clinical, anatomic/echocardiographic, and hemodynamic variables would predict procedural success and clinical outcome (Fig. 26.1). Demographic data, echocardiographic parameters (including echocardiographic score, Fig. 26.2), and procedure-related variables recorded from 1,085 consecutive patients who underwent PMV at the MGH and their long-term clinical follow-up (death, mitral valve replacement, redo PMV) were used to derive this clinical score. Multivariate regression analysis of the first 800 procedures was performed to identify independent predictors of procedural success. Significant variables were formulated into a risk score and validated prospectively. Six independent predictors of PMV success were identified: age less than 55 years, New York Heart Association classes I and II, pre-PMV mitral area of 1 cm2 or greater, pre-PMV mitral regurgitation grade less than 2, echocardiographic score of 8 or greater, and male sex. A score was constructed from the arithmetic sum of variables present per patient. Procedural success rates increased incrementally with increasing score (0 % for 0/6, 39.7 % for 1/6, 54.4 % for 2/6, 77.3 % for 3/6, 85.7 % for 4/6, 95 % for 5/6, and 100 % for 6/6; P < .001). In a validation cohort (n = 285 procedures), the multifactorial score remained a significant predictor of PMV success (P < .001). Comparison between the new score and the echocardiographic score confirmed that the new index was more sensitive and specific (P < .001). This new score also predicts long-term outcomes (P < .001). Clinical, anatomic, and hemodynamic variables predict PMV success and clinical outcome and may be formulated in a scoring system that would help to identify the best candidates for PMV.
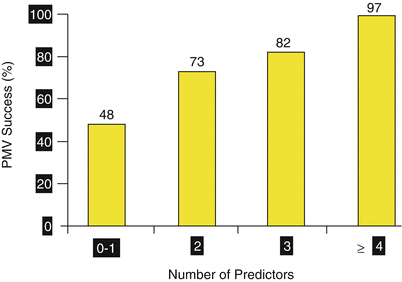
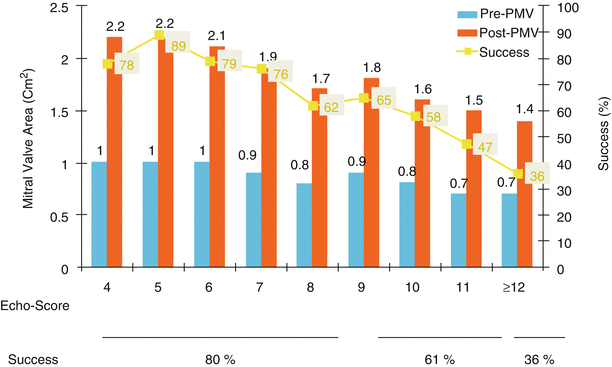

Fig. 26.1
A multifactorial score derived from clinical, anatomic/echocardiographic, and hemodynamic variables would predict procedural success and clinical outcome
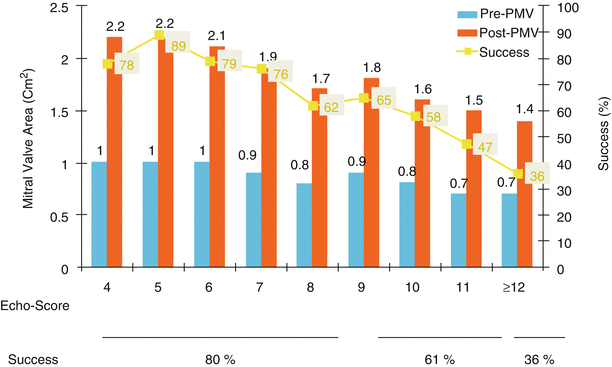
Fig. 26.2
Relationship between the echo score, the pre- and post-PMV MVA, and immediate success after PMV
PMV Technique
PMV is performed in the fasting state under mild sedation. Antibiotics (dicloxacillin 500 mg p.o. q/6 h for four doses started before the procedure or cefazolin 1 g i.v. at the time of the procedure) are used. Patients allergic to penicillin should receive vancomycin 1 g i.v. at the time of the procedure.
All patients carefully chosen as candidates for mitral balloon valvuloplasty should undergo diagnostic right and left and transseptal left heart catheterization. Following transseptal left heart catheterization, systemic anticoagulation is achieved by the intravenous administration of 100 units/Kg of heparin. In patients older than 40 years, coronary arteriography is recommended and should also be performed.
Hemodynamic measurements, cardiac output, and cine left ventriculography are performed before and after PMV. Cardiac output is measured by thermodilution and Fick method techniques. Mitral valve calcification and angiographic severity of mitral regurgitation (Sellers’ classification) are graded qualitatively from 0 to 4 grades as previously described [36]. An oxygen diagnostic run is performed before and after PMV to determine the presence of left to right shunt across the atrial septum after PMV.
There is not a unique technique of percutaneous mitral balloon valvuloplasty. Most of the techniques of PMV require transseptal left heart catheterization and use of the antegrade approach [12–22, 24–28]. Antegrade PMV can be accomplished using a single- (Fig. 26.3a) or a double-balloon technique (Fig. 26.3b). In this later approach, the two balloons could be placed through a single femoral vein and single transseptal punctures or through two femoral veins and two separate atrial septal punctures. In the retrograde technique of PMV, the balloon dilating catheters are advanced percutaneously through the right and left femoral arteries over guide wires that have been snared from the descending aorta. These guide wires have been advanced transseptally from the right femoral vein into the left atrium, the left ventricle, and the ascending aorta [37]. A retrograde nontransseptal technique of PMV has also been described [38]. Recently a technique of PMV using a newly designed metallic valvulotome was introduced [24]. The device consists of a detachable metallic cylinder with two articulated bars screwed onto the distal end of a disposable catheter whose proximal end is connected to an activating pliers. Squeezing the pliers opens the bars up to a maximum of 40 mm (Fig. 26.3c). The results with this device are at least comparable to those of the other balloon techniques of PMV [27]. However, multiple uses after sterilization should markedly decrease procedural costs.
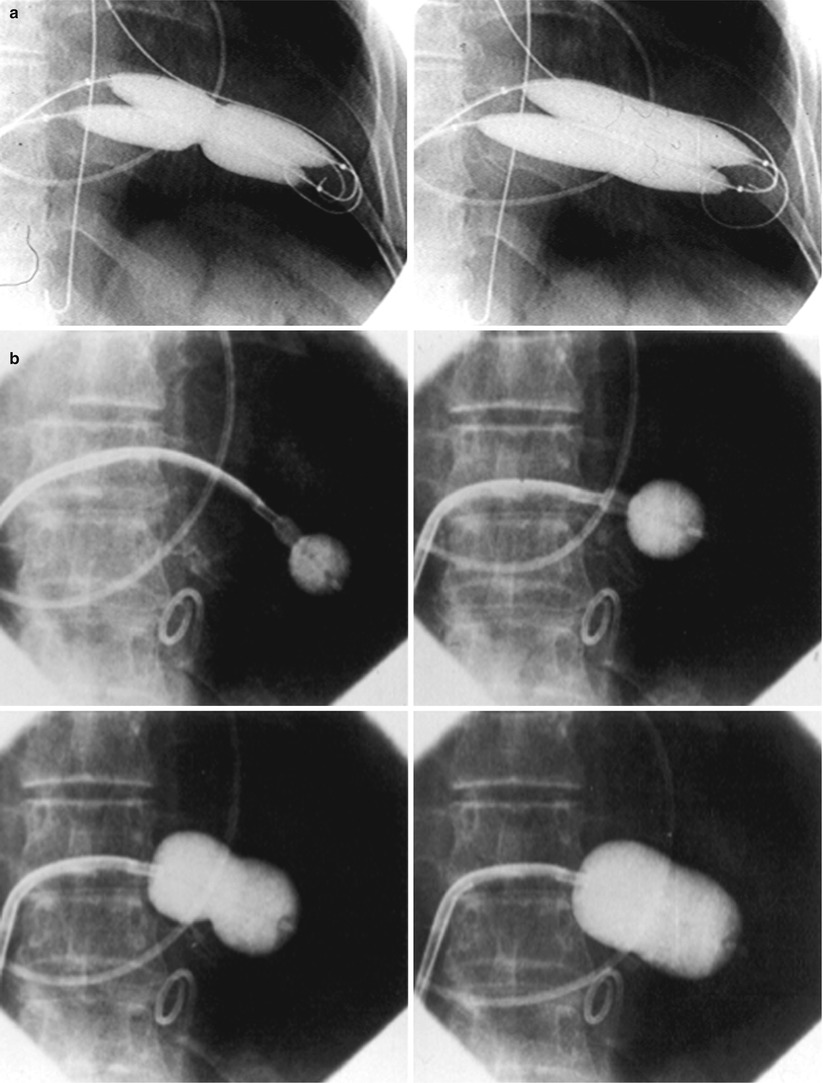
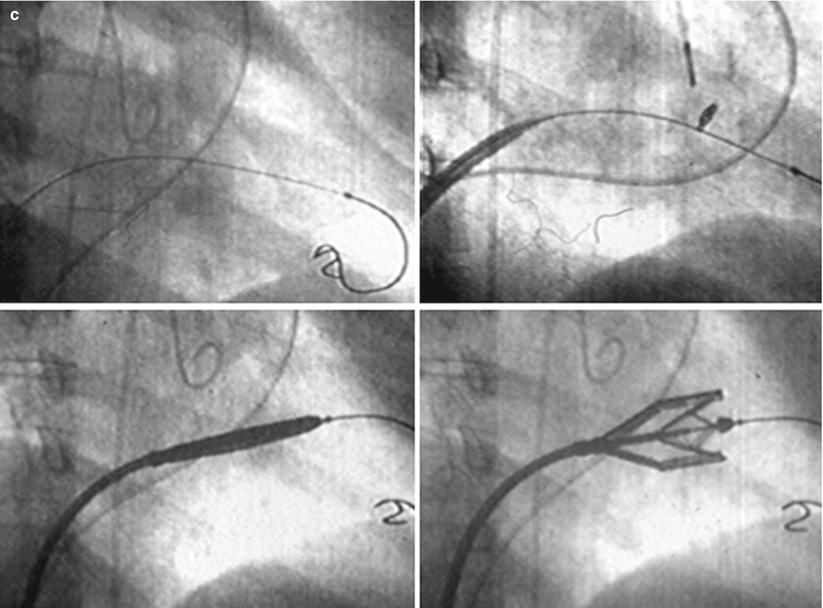


Fig. 26.3
Different percutaneous approaches of PMV: the double-balloon technique (a), the Inoue technique (b), and the metallic valvulotome (c)
The Antegrade Double-Balloon Technique
In performing PMV using the antegrade double-balloon technique (Fig. 26.3a), two 0.0038 in., 260 cm long Teflon-coated exchange wires are placed across the mitral valve into the left ventricle, through the aortic valve into the ascending and then the descending aorta [14–16]. Care should be taken to maintain large and smooth loops of the guide wires in the left ventricular cavity to allow appropriate placement of the dilating balloons. If a second guide wire cannot be placed into the ascending and descending aorta, a 0.038 in. Amplatz-type transfer guide wire with a preformed curlew at its tip can be placed at the left ventricular apex. In patients with aortic valve prosthesis, both guide wires with performed curlew tips should be placed at the left ventricular apex. When one or both guide wires are placed in the left ventricular apex, the balloons should be inflated sequentially. Care should be taken to avoid forward movement of the balloons and guide wires to prevent left ventricular perforation. Two balloon dilating catheters, chosen according to the patients’ body surface area, are then advanced over each one of the guide wires and positioned across the mitral valve parallel to the longitudinal axis of the left ventricle. The balloon valvotomy catheters are then inflated by hand until the indentation produced by the stenotic mitral valve is no longer seen. Generally one but occasionally two or three inflations are performed. After complete deflation, the balloons are removed sequentially.
The Inoue Technique of PMV
PMV can also been performed using the Inoue technique (Fig. 26.3b) [12]. The Inoue balloon is a 12 French shaft, coaxial, double-lumen catheter. The balloon is made of a double layer of rubber tubing with a layer of synthetic micromesh in between. Following transseptal catheterization, a stainless steel guide wire is advanced through the transseptal catheter and placed with its tip coiled into the left atrium and the transseptal catheter removed. A 14 French dilator is advanced over the guide wire and used to dilate the femoral vein and the atrial septum. A balloon catheter chosen according to the patient’s height is advanced over the guide wire into the left atrium. The distal part of the balloon is inflated and advanced into the left ventricle with the help of the spring wire stylet, which has been inserted through the inner lumen of the catheter. Once the catheter is in the left ventricle, the partially inflated balloon is moved back and forth inside the left ventricle to assure that it is free of the chordae tendineae. The catheter is then gently pulled against the mitral plane until resistance is felt. The balloon is then rapidly inflated to its full capacity and then deflated quickly. During inflation of the balloon, an indentation should be seen in its mid-portion. The catheter is withdrawn into the left atrium and the mitral gradient and cardiac output measured. If further dilatations are required, the stylet is introduced again and the sequence of steps described above repeated at a larger balloon volume. After each dilatation, its effect should be assessed by pressure measurement, auscultation, and 2D echocardiography. If mitral regurgitation occurs, further dilation of the valve should not be performed.
Mechanism of PMV
The mechanism of successful PMV is splitting of the fussed commissures toward the mitral annulus, resulting in commissural widening. This mechanism has been demonstrated by pathological, surgical, and echocardiographic studies [39–43]. In addition in patients with calcific mitral stenosis, the balloons could increase mitral valve flexibility by the fracture of the calcified deposits in the mitral valve leaflets [44]. Although rare, undesirable complications such as leaflet tears, left ventricular perforation, tear of the atrial septum, and rupture of chordae, mitral annulus, and papillary muscle could also occur.
Immediate Outcomes
Figure 26.3 shows the hemodynamic changes produced by PMV in one patient. PMV resulted in a significant decrease in mitral gradient, mean left atrium pressure, and mean pulmonary artery pressure and an increase in cardiac output and mitral valve area. Table 26.2 shows the changes in mitral valve area reported by several investigators using different techniques of PMV. In most series, PMV is reported to increase mitral valve area from less than 1.0 cm2 to approximately 2.0 cm2 [18–20, 23, 25–28, 34].
Table 26.2
Immediate changes in mitral valve area after percutaneous mitral valvuloplasty (PMV)
Author | Institution | No. patients | Age | Pre-PMV | Post-PMV |
|---|---|---|---|---|---|
Palacios et al. | MGHa | 879 | 55 ± 15 | 0.9 ± 0.3 | 1.9 ± 0.7 |
Vahanian et al. | Tenon | 1,514 | 45 ± 15 | 1.0 ± 0.2 | 1.9 ± 0.3 |
Hernández et al. | Clínico Madrid | 561 | 53 ± 13 | 1.0 ± 0.2 | 1.8 ± 0.4 |
Stefanadis et al. | Athens University | 438 | 44 ± 11 | 1.0 ± 0.3 | 2.1 ± 0.5 |
Chen et al. | Guangzhou | 4,832 | 37 ± 12 | 1.1 ± 0.3 | 2.1 ± 0.2 |
NHLBI | Multicenter | 738 | 54 ± 12 | 1.0 ± 0.4 | 2.0 ± 0.2 |
Inoue et al. | Takeda | 527 | 50 ± 10 | 1.1 ± 0.1 | 2.0 ± 0.1 |
Inoue Registry | Multicenter | 1,251 | 53 ± 15 | 1.0 ± 0.3 | 1.8 ± 0.6 |
Ben Farhat et al. | Fattouma | 463 | 33 ± 12 | 1.0 ± 0.2 | 2.2 ± 0.4 |
Arora et al. | G.B. Pan | 600 | 27 ± 8 | 0.8 ± 0.2 | 2.2 ± 0.4 |
Cribier et al. | Ruen | 153 | 36 ± 15 | 1.0 ± 0.2 | 2.2 ± 0.4 |
Eight hundred and seventy-nine consecutive patients with mitral stenosis have undergone 939 PMVs at the Massachusetts General Hospital between July 1986 and July 2000 [28]. As shown in Fig. 26.4, in this group of patients, PMV resulted in a significant decrease in mitral gradient from 14 ± 6 to 6 ± 3 mmHg. The mean cardiac output significantly increased from 3.9 ± 1.1 to 4.5 ± 1.3 L/min. and the calculated mitral valve area from 0.9 ± 0.3 to 1.9 ± 0.7 cm2. In addition, mean pulmonary artery pressure significantly decreased from 36 ± 13 to 29 ± 11 mmHg and the mean left atrial pressure decreased from 25 ± 7 to 17 ± 7 mmHg and, consequently, the calculated pulmonary vascular resistance decreased significantly following PMV.
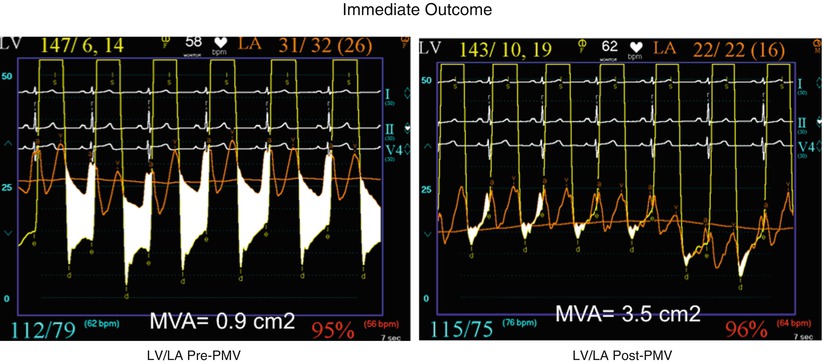

Fig. 26.4
Hemodynamic changes produced by a successful PMV in one patient with severe mitral stenosis. At the top panels, simultaneous left atrium and left ventricular pressures before (upper-right panel) and after (upper-left panel) PMV. The phasic and mean pulmonary artery pressures before (lower-right panel) and after (lower-left panel) PMV are shown at the bottom. The corresponding cardiac outputs and calculated mitral valve areas are also displayed (LV left ventricle, LA left atrium, PA pulmonary artery pressure, PMV percutaneous mitral balloon valvuloplasty)
A successful hemodynamic outcome (defined as a post-PMV mitral valve area ≥1.5 cm2 and post-PMV MR <3 Sellers’ grade) was obtained in 72 % of the patients. Although a suboptimal result occurred in 28 % of the patients, a post-PMV mitral valve area ≤1.0 cm2 (critical mitral valve area) was present in only 8.7 % of these patients.
Predictors of Increase in Mitral Valve Area and Procedural Success
Univariate analysis demonstrated that the increase in mitral valve area with PMV is directly related to the balloon size employed as it reflects in the effective balloon dilating area (EBDA) and inversely related to the echocardiographic score (Fig. 26.2), the presence of atrial fibrillation, the presence of fluoroscopic calcium, the presence of previous surgical commissurotomy, older age, NYHA pre-PMV, and presence of mitral regurgitation before PMV. Multiple stepwise regression analysis identified balloon size (p < .02), the echocardiographic score (p < .0001), and the presence of atrial fibrillation (p < .009) and mitral regurgitation before PMV (p < 0.03) as independent predictors of the increase in mitral valve area with PMV.
Univariate predictors of procedural success included age, pre-PMV MVA, mean pre-PMV pulmonary artery pressure, male sex, echocardiographic score, pre-PMV mitral regurgitation ≥2+, history of previous surgical commissurotomy, presence of atrial fibrillation, and presence of mitral valve calcification under fluoroscopy.
Multiple stepwise logistic regression analysis identified larger pre-PMV MVA (odds ratio [OR] 13.05, 95 % confidence interval [CI]: 7.74–22.51; p < 0.001), less degree of pre-PMV mitral regurgitation (OR 3.85, CI: 2.27–6.66; p < 0.001), younger age (OR 3.33, CI: 1.41–7.69; p = 0.006), absence of previous surgical commissurotomy (OR 1.85, CI: 1.20–2.86; p = 0.004), male sex (OR 1.92, CI: 1.19–3.13; p = 0.008), and echocardiographic score ≤8 (OR 1.69, CI: 1.18–2.44; p = 0.004).
The Echocardiographic Score
The echocardiographic examination of the mitral valve can accurately characterize the severity and extent of the pathological process in patients with mitral stenosis. The most utilized score to identify the anatomic abnormalities of the stenotic mitral valve is that described by Wilkins et al. (Table 26.3, Fig. 26.2) [42]. This echocardiographic score is an important predictor of the immediate and long-term outcome of PMV. In this morphologic score, each of the following: leaflet rigidity, leaflet thickening, valvular calcification, and subvalvular disease, is scored from 0 to 4. A higher score would represent a heavily calcified, thicken, and immobile valve with extensive thickening and calcification of the subvalvular apparatus. The increase in mitral valve area with PMV is inversely related to the echocardiographic score. The best outcome with PMV occurs in those patients with echocardiographic scores ≤8. The increase in mitral valve area is significantly greater in patients with echocardiographic scores ≤8 than in those with echocardiographic score >8. Among the four components of the echocardiographic score, valve leaflets thickening and subvalvular disease correlate the best with the increase in mitral valve area produced by PMV [44]. Therefore, suboptimal results with PMV are more likely to occur in patients with valves which are more rigid, more thickened, and those with more subvalvular fibrosis and calcification.
Table 26.3
The echocardiographic score. Echocardiographic grading of the severity and extent of the anatomic abnormalities in patients with mitral stenosis
Grade | Leaflet mobility | Valvular thickening | Valvular calcification | Subvalvular thickening |
|---|---|---|---|---|
0 | Normal | Normal | Normal | Normal |
1 | Highly mobile valve with restriction of only the leaflet tips | Leaflet near normal (4–5 mm) | A single area of increased echo brightness | Minimal thickening of chordal structures just below the valve |
2 | Middle portion and base of leaflets have reduced mobility | Mid-leaflet thickening, marked thickening of the margins | Scattered areas of brightness confined to leaflet margins | Thickening of chordae extending up to one third of chordal length |
3 | Valve leaflets move forward in diastole mainly at the base | Thickening extending through the entire leaflets (5–8 mm) | Brightness extending into the mid-portion of leaflets | Thickening extending to the distal third of the chordae |
4 | No or minimal forward movement of the leaflets in diastole | Marked thickening of all leaflet tissue (>8–10 mm) | Extensive brightness throughout most of the leaflet tissue | Extensive thickening and shortening of all chordae extending down to the papillary muscles |
Balloon Size and EBDA
The increase in mitral valve area with PMV is directly related to balloon size. This effect was first demonstrated in a subgroup of patients who underwent repeat PMV [45]. They initially underwent PMV with a single balloon resulting in a mean mitral valve area of 1.2 ± 0.2 cm2. They underwent repeat PMV using the double-balloon technique, which increased the effective balloon dilating area (EBDA) normalized by body surface area (EBDA/BSA) from 3.41 ± 0.2 to 4.51 ±0.2 cm2/m2. The mean mitral valve area in this group after repeat PMV was 1.8 ± 0.7 cm2. The increase in mitral valve area in patients who underwent PMV at the Massachusetts General Hospital using the double-balloon technique (EBDA of 6.4 ± 0.03 cm2) was significantly greater than the increase in mitral valve area achieved in patients who underwent PMV using the single-balloon technique (EBDA of 4.3 ± 0.02 cm2). The mean mitral valve areas were 1.9 ± 0.7 cm2 and 1.4 ± 0.1 cm2 for patients who underwent PMV with the double-balloon and the single-balloon techniques, respectively. However, care should be taken in the selection of dilating balloon catheters so as to obtain an adequate final mitral valve area and no change or a minimal increase in mitral regurgitation.
Mitral Valve Calcification
The immediate outcome of patients undergoing PMV is inversely related to the severity of valvular calcification seen by fluoroscopy. Patients without fluoroscopic calcium have a greater increase in mitral valve area after PMV than patients with calcified valves. Patients with either no or 1 + fluoroscopic calcium have a greater increase in mitral valve area after PMV (1.1 ± 0.6 cm2 and 0.9 ± 0.5 cm2, respectively) than those patients with 2, 3, or 4 + of calcium (0.8 ± 0.6 cm2, 0.8 ± 0.5 cm2, and 0.6 ± 0.4 cm2, respectively).
Previous Surgical Commissurotomy
Although the increase in mitral valve area with PMV is inversely related to the presence of previous surgical mitral commissurotomy, PMV can produce a good outcome in this group of patients. The post-PMV mean mitral valve area in 154 patients with previous surgical commissurotomy was 1.8 ± 0.7 cm2 compared with a valve area of 1.9 ± 0.6 cm2 in patients without previous surgical commissurotomy (P < 0.05). In this group of patients, an echocardiographic score ≤8 was an important predictor of a successful hemodynamic immediate outcome.
Age
The immediate outcome of PMV is directly related to the age of the patient. The percentage of patients obtaining a good result with this technique decreases as age increases. A successful hemodynamic outcome from PMV was obtained in only less than 50 % of patients ≥65 years old [46]. This inverse relationship between age and the immediate outcome from PMV is due to the higher frequency of atrial fibrillation, calcified valves, and higher echocardiographic scores in elderly patients.
Atrial Fibrillation
The increase in mitral valve area with PMV is inversely related to the presence of atrial fibrillation; the post-PMV mitral valve area of patients in normal sinus rhythm was 2.0 ± 0.7 cm2 compared with a valve area of 1.7 ± 0.6 cm2 of those patients in atrial fibrillation. The inferior immediate outcome of PMV in patients with mitral stenosis who are in atrial fibrillation is more likely related to the presence of clinical and morphological characteristics associated with inferior results after PMV. Patients in atrial fibrillation are older and present more frequently with echocardiographic scores >8, NYHA functional class IV, calcified mitral valves under fluoroscopy, and with a previous history of surgical mitral commissurotomy.
Mitral Regurgitation Before PMV
The presence and severity of mitral regurgitation before PMV is an independent predictor of unfavorable outcome. The increase in mitral valve after PMV is inversely related to the severity of mitral regurgitation determined by angiography before the procedure. This inverse relationship between presence of mitral regurgitation and immediate outcome of PMV is in part due to the higher frequency of atrial fibrillation, higher echocardiographic scores, calcified mitral valves under fluoroscopy, and older age in patients with mitral regurgitation before PMV.
Complications
Table 26.4 shows the complications reported by several investigators after PMV [18–20, 23, 25–28, 34]. Mortality and morbidity with PMV are low and similar to surgical commissurotomy. Overall, there is less than 1 % mortality. Severe mitral regurgitation (4 grades by angiography) has been reported in 1–5.2 % of the patients. Some of these patients required inhospital mitral valve replacement. Thromboembolic episodes and stroke have been reported in 0–3.1 % and pericardial tamponade in 0.2–4.6 % of cases in these series. Pericardial tamponade can occur from transseptal catheterization and more rarely from ventricular perforation. PMV is associated with a 3–16 % incidence of left to right shunt immediately after the procedure. However, the pulmonary to systemic flow ratio is ≥2:1 in only a minimum number of patients.
Table 26.4
Complications following percutaneous mitral valvuloplasty
Author | No. patients | Mortality (%) | Tamponade (%) | Severe MRa (%) | Embolism (%) |
|---|---|---|---|---|---|
Palacios et al. | 879 | 0.6 | 1.0 | 3.4 | 1.8 |
Vahanian et al. | 1,514 | 0.4 | 0.3 | 3.4 | 0.3 |
Hernández et al. | 561 | 0.4 | 0.6 | 4.5 | |
Stefanadis et al. | 438 | 0.2 | 0.0 | 3.4 | 0.0 |
Chen et al. | 4,832 | 0.1 | 0.8 | 1.4 | 0.5 |
NHLBI | 738 | 3.0 | 4.0 | 3.0 | 3.0 |
Inoue et al. | 527 | 0.0 | 1.6 | 1.9 | 0.6 |
Inoue Registry | 1,251 | 0.6 | 1.4 | 3.8 | 0.9 |
Ben Farhat et al. | 463 | 0.4 | 0.7 | 4.6 | 2.0 |
Arora et al. | 600 | 1.0 | 1.3 | 1.0 | 0.5 |
Cribier et al. | 153 | 0.0 | 0.7 | 1.4 | 0.7 |
We have demonstrated that severe mitral regurgitation (4 grades by angiography) occurs in about 3 % of patients undergoing PMV [28]. An undesirable increase in mitral regurgitation (≥2 grades by angiography) occurred in 10.1 % of patients. This undesirable increase in mitral regurgitation is well tolerated in most patients. Furthermore, more than half of them have less mitral regurgitation at follow-up cardiac catheterization. We have demonstrated that the ratio of the effective balloon dilating area to body surface area (EBDA/BSA) is the only predictor of increased mitral regurgitation after PMV [47–49]. The effective balloon dilating area (EBDA) is calculated using standard geometric formulas. The incidence of mitral regurgitation is lower if balloon sizes are chosen so that EBDA/BSA is ≤4.0 cm2/m2. The single-balloon technique results in a lower incidence of mitral regurgitation but provides less relief of mitral stenosis than the double-balloon technique. Thus, there is an optimal effective balloon dilating area between 3.1 and 4.0 cm2/m2 which achieves a maximal mitral valve area with a minimal increase in mitral regurgitation. An echocardiographic score for the mitral valve that can predict the development of severe mitral regurgitation following PMV has also been described [43]. This score takes into account the distribution (even or uneven) of leaflet thickening and calcification, the degree and symmetry of commissural disease, and the severity of subvalvular disease.
Left to right shunt through the created atrial communication occurred in 3–16 % of the patients undergoing PMV. The size of the defect is small as reflected in the pulmonary to systemic flow ratio of <2:1 in the majority of patients. Older age, fluoroscopic evidence of mitral valve calcification, higher echocardiographic score, pre-PMV lower cardiac output, and higher pre-PMV NYHA functional class are the factors that predispose patients to develop left to right shunt post-PMV [49]. Clinical, echocardiographic, surgical, and hemodynamic follow-up of patients with post-PMV left to right shunt demonstrated that the defect closed in approximately 60 %. Persistent left to right shunt at follow-up is small (QP/QS < 2:1) and clinically well tolerated. In the series from the Massachusetts General Hospital, there is one patient in whom the atrial shunt remained hemodynamically significant at follow-up. This patient underwent percutaneous transcatheter closure of her atrial defect with a clamshell device. Desideri et al. reported atrial shunting determined by color flow transthoracic echocardiography in 61 % of 57 patients immediately after PMV. The shunt persisted in 30 % of patients at 19 ± 6 (range 9–33) months follow-up [50]. They identified the magnitude of the post-PMV atrial shunt (QP/QS > 1.5:1) and use of bifoil balloon (2 balloons on 1 shaft) and smaller post-PMV mitral valve area as independent predictors of the persistence of atrial shunt at long-term follow-up.
Clinical Follow-Up
Long-term follow-up studies after PMV are encouraging [21–23, 25, 26, 28, 34]. Following PMV, the majority of patients have marked clinical improvement and become NYHA classes I or II. The symptomatic, echocardiographic, and hemodynamic improvement produced by PMV persists in intermediate and long-term follow-up. The best long-term results are seen in patients with echocardiographic scores ≤8. When PMV produces a good immediate outcome in this group of patients, restenosis is unlikely to occur at follow-up. Although PMV can result in a good outcome in patients with echocardiographic scores >8, hemodynamic and echocardiographic restenosis is frequently demonstrated at follow-up despite ongoing clinical improvement. Table 26.5 shows long-term follow-up results of patients undergoing PMV at different institutes. We reported an estimated 12-year survival rate of 74 % in a cohort of 879 patients undergoing PMV at the Massachusetts General Hospital (Fig. 26.5). Death at follow-up was directly related to age, post-PMV pulmonary artery pressure, and pre-PMV NYHA functional class IV. In the same group of patients, the 12-year event-free survival (alive and free of mitral valve replacement or repair and redo PMV) was 33 % (Fig. 26.6). Cox regression analysis identified age (risk ratio (R.R.) 1.02; C.I. 1.01–1.03; p < 0.0001), pre-PMV NYHA functional class IV (R.R. 1.35; C.I. 1.00–1.81; p = 0.05), prior commissurotomy (R.R. .150; C.I. 1.16–1.92; p = 0.002), the echocardiographic score (R.R. 1.31; C.I. 1.02–1.67; p = 0.003), pre-PMV mitral regurgitation ≥2+ (R.R. 1.56; C.I. 1.09–2.22; p = 0.02), post-PMV mitral regurgitation ≥3+ (R.R. 3.54; C.I. 2.61–4.72; p < 0.0001), and post-PMV mean pulmonary artery pressure (R.R 1.02; C.I 1.01–1.03; p < 0.0001) as independent predictors of combined events at long-term follow-up.
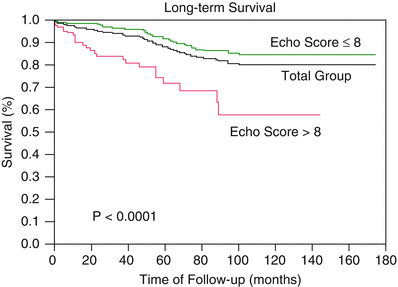
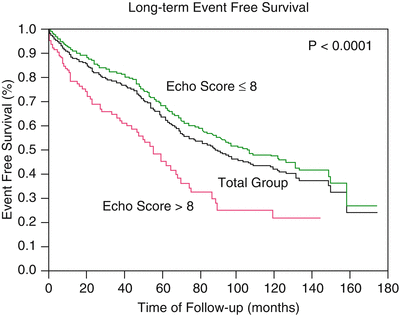
Table 26.5
Clinical long-term follow-up after percutaneous mitral valvuloplasty
Author | No. patients | Age (years) | Follow-up | Survival (%) | Event-free survival (%) |
|---|---|---|---|---|---|
Palacios et al. | 879 | 55 | 12 | 74 | 33 |
Lung et al. | 1,024 | 49 | 10 | 85 | 56 |
Hernández et al. | 561 | 53 | 7 | 95 | 69 |
Orrange et al. | 132 | 44 | 7 | 83 | 65 |
Ben Farhat et al. | 30 | 29 | 7 | 100 | 90 |
Stefanadis et al. | 441 | 44 | 9 | 98 | 75 |

Fig. 26.5
Fifteen-year survival for all patients and for patients with echocardiographic score ≤8 and >8, undergoing percutaneous mitral balloon valvotomy at the Massachusetts General Hospital

Fig. 26.6
Fifteen-year event-free survival for all patients and for patients with echocardiographic score ≤8 and >8, undergoing percutaneous mitral balloon valvotomy at the Massachusetts General Hospital
Actuarial survival and event-free survival rates throughout the follow-up period were significantly better in patients with echocardiographic scores ≤8. Survival rates were 82 % for patients with echocardiographic score ≤8 and 57 % for patients with score >8 at a follow-up time of 12 years (p < 0.0001). Event-free survival (38 % vs. 22 %; p < 0.0001) at 12-year follow-up was also significantly higher for patients with echocardiographic score ≤8. Similar follow-up studies have been reported in other series with the double-balloon technique and with the Inoue technique of PMV [21, 23, 25, 26]. Over 90 % of young patients with pliable valves in sinus rhythm and with no evidence of calcium under fluoroscopy remain free of cardiovascular events at an approximately follow-up of 5 years [17, 22, 34].
Functional deterioration at follow-up is late and related primarily to mitral restenosis [26]. The incidence of restenosis, as assessed by sequential echocardiography, is approximately 40 % after 7 years [25]. Repeat PMV can be proposed if recurrent stenosis leads to symptoms. At the moment, we have only a small number of series available on redo PMV. They show encouraging results in selected patients with favorable characteristics when restenosis occurs several years after an initially successful procedure and if the predominant mechanism of restenosis is commissural refusion [45].
Follow-Up in the Elderly
Tuzcu et al. reported the outcome of PMV in 99 elderly patients (≥ 65 year) [46]. A successful outcome (valve area ≥1.5 cm2 without ≥2+ increase in mitral regurgitation and without left to right shunt of ≥1.5 : 1) was achieved in 46 patients. The best multivariate predictor of success was the combination of echocardiographic score, NYHA functional class, and inverse of mitral valve area. Patients who had an unsuccessful outcome from PMV were in a higher NYHA functional class and had higher echocardiographic scores and smaller mitral valve areas pre-PMV compared to those patients who had a successful outcome. Actuarial survival and combined event-free survival at 3 years were significantly better in the successful group. Mean follow-up was 16 ± 1 months. Actuarial survival (79 ± 7 % vs. 62 ± 10 %; p = .04), survival without mitral valve replacement (71 ± 8 % vs. 41 ± 8 %; p = .002), and event-free survival (54 ± 12 % vs. 38 ± 8 %; p = .01) at 3 years were significantly better in the successful group of 46 patients than the unsuccessful group of 53 patients. Low echocardiographic score was the independent predictor of survival, and the lack of mitral valve calcification was the strongest predictor of event-free survival.
Recently data reported in 96 patients ≥75 years have shown that these patients present a lower pre-PMV mitral valve area (0.8 ± 0.3 vs. 0.9 ± 0.3; p = 0.005), a lower post-PMV mitral valve area (1.6 ± 0.6 vs. 1.9 ± 0.7; p < 0.0001), and a lower procedural success (51.0 % vs. 71.4 %; p < 0.0001) compared with patients <75 years [51]. Patients ≥75 years exhibited a higher inhospital mortality than patients <75 years (3.1 % vs. 0.3 %) with no significant differences in the other procedure-related complications (cardiac tamponade, severe mitral regurgitation, significant left to right shunt, and embolism). Although inhospital mortality was higher, in the majority of these patients, PMV was considered as a palliative treatment. However, technical complications were similar to those more favorable patients aged <75. Survival and event-free survival rates were 60 and 49 % for patients ≥75 years at a follow-up time of 3 years. The echo score is an imperfect predictor of hemodynamic improvement in elderly patients [51, 52].
Unfortunately, no randomized study is available for elderly patients, and a comparison of the results of PMV with those of surgical series is difficult because of the differences in the patients and surgical techniques involved.
Follow-Up of Patients with Calcified Mitral Valves
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree