and Yaowanuj Kirilak2
(1)
Institute of Soil and Water Conservation, Northwest A&F University, Shaanxi, China
(2)
Centre for Microscopy, Characterisation and Analysis, The University of Western Australia, Crawley, WA, Australia
Abstract
This chapter describes protocols using formalin-acetic acid-alcohol (FAA) to fix plant tissues for studying biomineralization by means of scanning electron microscopy (SEM) and qualitative energy-dispersive X-ray microanalysis (EDX). Specimen preparation protocols for SEM and EDX mainly include fixation, dehydration, critical point drying (CPD), mounting, and coating. Gold-coated specimens are used for SEM imaging, while gold- and carbon-coated specimens are prepared for qualitative X-ray microanalyses separately to obtain complementary information on the elemental compositions of biominerals. During the specimen preparation procedure for SEM, some biominerals may be dislodged or scattered, making it difficult to determine their accurate locations, and light microscopy is used to complement SEM studies. Specimen preparation protocols for light microscopy generally include fixation, dehydration, infiltration and embedding with resin, microtome sectioning, and staining. In addition, microwave processing methods are adopted here to speed up the specimen preparation process for both SEM and light microscopy.
Key words
BiomineralizationCritical point dryingDehydrationEnergy-dispersive X-ray microanalysisFAAGMA embeddingLight microscopyMicrowavePlant tissuesScanning electron microscopy1 Introduction
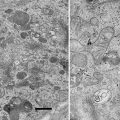
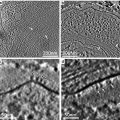
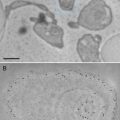
Biomineralization, the formation of minerals by living organisms, is common in the plant kingdom. Most biominerals in plant tissues have been identified as calcium oxalate, and their proposed functions include bulk element (mainly calcium) regulation, detoxification of aluminum and heavy metals such as strontium and cadmium, and defense against herbivory. Functions of biominerals in plants may be variable, depending on their locations, elemental compositions, shapes, and sizes [1].
The locations of most biominerals can be identified through preserving plant tissues in appropriate fixatives, dehydrating, resin or paraffin infiltrating and embedding, cutting specimens into semi-thin sections, staining sections, and observing the sections under a polarizing microscope or a bright-field microscope [2–5]. However, not all biominerals can be identified using either a polarizing or bright-field microscope [6, 7]. In addition, elemental compositions of these biominerals cannot be determined by using light microscopy techniques.
Using scanning electron microscopy (SEM) permits one to study the morphology and surface details of solid materials, including biominerals in plant tissues. For the fundamental information on SEM, the reader is referred to the literature [8]. Energy-dispersive X-ray microanalysis (EDX) conducted in a scanning electron microscope allows the elemental analysis of bulk or semi-thick specimens. The basis of EDX in a SEM is that the electron beam scans across the specimen and excites atoms to emit X-ray photons, which are collected by a semiconductor crystal. The energy of X-ray photon hitting the crystal is converted into a voltage pulse proportional to the energy of the X-ray photon. Each element has its characteristic X-ray(s) and voltage pulse(s), allowing the element present to be identified. The theories of EDX are explained in details in the literature [9, 10].
SEM and qualitative EDX have been widely applied in studying biomineralization. Using SEM has helped numerous researchers to study the morphologies and locations of biominerals in plant tissues [6, 7, 11–16], while EDX conducted in a SEM permits the determination of elemental compositions of biominerals [6, 7, 12–16]. However, some biominerals may be dislodged or scattered during the specimen preparation procedure for SEM, making it difficult to determine their accurate locations. Sometimes light microscopy and SEM can complement each other. This chapter will focus on the specimen preparation protocols for studying biomineralization in plant tissues by means of SEM and EDX using formalin-acetic acid-alcohol (FAA) as the fixative and also includes some information on specimen preparation protocols using glutaraldehyde as the fixative for light microscopy.
Specimen preparation for SEM and EDX follows the same protocols, i.e., fixation, dehydration, critical point drying (CPD), mounting, and coating. For light microscopy, plant tissues are often difficult to fix and infiltrate using conventional protocols, especially for old and hard tissues, and those having biominerals. Microwave processors have been widely used in processing biological samples (including plant tissues) for microscopy work, and in many cases, microwave-assisted processing has been proved to significantly reduce processing times and improve specimen morphology [17–19]. To process very hard plant tissues which usually require particularly long time, conventional protocols can be combined with microwave-assisted processing protocols.
2 Materials
2.1 SEM and EDX
2.1.1 Equipments
1.
Fine-tipped forceps and SEM mount forceps.
2.
Microwave processor (Pelco 34700 Biowave® Microwave Tissue Processor, Ted Pella, Inc., Redding, CA).
3.
Critical point dryer (Polaron E3000, Quorum Technologies, Ashford, Kent, UK).
4.
Sputter coater (Polaron E 5000, Quorum Technologies, Ashford, Kent, UK) or vacuum evaporation coating unit (Speedivac coating unit, model 12E6/1167, Edwards High Vacuum Ltd., Crawley, West Sussex, UK).
5.
Scanning electron microscope (Zeiss 1555 VP-FESEM, Carl Zeiss, Oberkochen, Germany) equipped with an energy-dispersive X-ray detector (Oxford Instruments, Oxford, UK).
6.
Computer with software (e.g., AZtec and INCA software) for acquiring and processing spectra.
2.1.2 Consumables
1.
Double-edged razor blades.
2.
Plastic Petri dishes.
3.
Whatman No. 1 90-mm filter paper.
4.
5-mL microwave-safe plastic specimen vials.
5.
Plastic pipettes.
6.
Aluminum SEM mounting stubs.
7.
Double-sided sticky carbon tape.
8.
Carbon ink.
9.
Sharpened wooden sticks for painting carbon ink on specimens.
10.
SEM specimen support holder.
2.2 Light Microscopy
2.2.1 Equipments
1.
Vacuum oven with an attached vacuum gauge for specimen initial fixation and final polymerization.
2.
Microwave processor (Pelco 34700 Biowave® Microwave Tissue Processor, Ted Pella, Inc., Redding, CA).
3.
Rotating mixer (homemade).
4.
Hacksaw.
5.
Glass-knife maker (Leica EM KMR2 knife maker, Leica Microsystems, Wetzlar, Germany).
6.
Sorvall semi-automatic microtome, which can cut semi-thin sections.
7.
Hot plate.
8.
Bright-field microscope (Zeiss Axioplan Microscope, Carl Zeiss, Oberkochen, Germany) and polarizing microscope (Olympus BX43 Upright Microscope, Olympus Corporation, Tokyo, Japan).
2.2.2 Consumables
1.
Single- and double-edged razor blades.
2.
Plastic Petri dishes.
3.
Whatman No. 1 90-mm filter paper.
4.
5-mL microwave-safe plastic specimen vials.
5.
Plastic pipettes.
6.
Sharpened wooden sticks for specimen transfer.
7.
Soft tissue paper.
8.
Aluminum weighing dishes or peel-away embedding molds.
9.
Clean glass strips.
2.2.3 Chemicals
1.
0.1 M phosphate-buffered saline (PBS), pH 7.4.
2.
2.5 % (v/v) glutaraldehyde in 0.1 M PBS at pH 7.4.
3.
Absolute (100 %) acetone and increasing concentrations of acetone in distilled water (25, 50, 75, 90 %).
5.
0.05 % (w/v) toluidine blue O in benzoate buffer at pH 4.4.
3 Methods
3.1 SEM and EDX
3.1.1 Specimen Fixation
1.
Rinse plant tissues in tap water to remove dust, then cut fresh tissues into small sections (3–5 mm long) using double-edged razor blades in a Petri dish with a piece of filter paper wetted with FAA fixative (see Note 1).
2.
Transfer specimens into 5-mL microwave-safe plastic vials containing the same fixative using a pair of fine-tipped forceps or a sharpened wooden stick.
3.1.2 Dehydration and CPD
1.




Replace FAA with 70, 90, 95, and 100 % ethanol sequentially; the specimens are treated with microwave at 250 W power without vacuum for 3 min (1 min on, 1 min off, 1 min on), 2× for each ethanol gradient (see Notes 3 and 4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree