Applications for Fluorodeoxyglucose PET and PET/CT in the Evaluation of Patients with Colorectal Carcinoma
Dominique Delbeke
Colorectal cancer is the third most common cause of cancer in men and women, and it affects 5% of the population in the United States and most Western countries. The American Cancer Society estimates that there are approximately 135,000 new cases of colorectal cancer per year in the United States, and approximately 57,000 patients per year die from this disease in the United States, representing 10% of all cancer deaths. Approximately 70% to 80% of patients are treated with curative intent, and the overall survival at 5 years after diagnosis is less than 60%.
Screening and Diagnosis of Colorectal Carcinoma
The diagnosis of colorectal carcinoma is based on colonoscopy and biopsy. The American Cancer Society recommends screening for colorectal cancer with yearly fecal occult blood test and flexible sigmoidoscopy every 5 years after the age of 50 for asymptomatic individuals (1). Colonoscopy evaluating the entire colon is a more comprehensive screening examination and has also been advocated in patients over 50. The role of virtual colonoscopy with multidetector computed tomography (CT) is still debated (2), but this screening technique will likely grow in prominence. Although fluorodeoxyglucose (FDG) positron emission tomography (PET) is not routinely used for screening or diagnostic purposes, it is not uncommon to incidentally detect colorectal carcinoma on whole-body studies performed for other indications.
Accurate interpretation of FDG PET images requires knowledge of the normal physiological distribution of FDG. Uptake in the gastrointestinal tract is variable from patient to patient. For example, FDG uptake along the esophagus is common, especially in the distal portion and at the gastroesophageal junction and in the presence of esophagitis. The wall of the stomach is usually faintly seen and can be used as an anatomical landmark, but occasionally the uptake can be relatively intense. There is FDG uptake in the cecum of many patients that may be related to abundant lymphoid tissue in the intestinal wall, among other factors. When marked activity is present in the bowel, evaluation for recurrence at the anastomotic site can be masked. Mild to moderate uptake is also usually seen at colostomy sites.
FDG uptake normally present in the gastrointestinal tract can occasionally be difficult to differentiate from a malignant lesion. Incidental colonic FDG uptake in 27 patients without colorectal carcinoma was correlated with colonoscopic and/or histopathologic findings (3). Diffuse uptake in eight patients was normal and associated with a normal colonoscopy. Segmental uptake was due to colitis in five of six patients. Focal uptake in seven patients was associated with benign adenomas. A study of 110 patients has demonstrated that precancerous adenomatous polyps can be detected incidentally on whole body images performed for other indications with a sensitivity of 24% (24 of 59). The positivity rate increased to 90% for lesions greater than 13 mm in size, and the false-positive rate was 5.5% (six of ten) (4).
Agress and Cooper (5) reviewed FDG PET studies of 1,750 patients referred for evaluation of known or suspected malignancies. They found 58 unexpected focal areas of FDG uptake that were unlikely to be related to the known primary in 53 (3.3%) patients. Forty-two lesions were pathologically confirmed and 30 of 42 (71%) were malignant or premalignant, including 18 colonic adenomas and 3 colon carcinomas. Similar data were published by Kamel et al. (6) on a review of 3,281 patients and Gutman et al. (7) on a review of 1,716 patients.
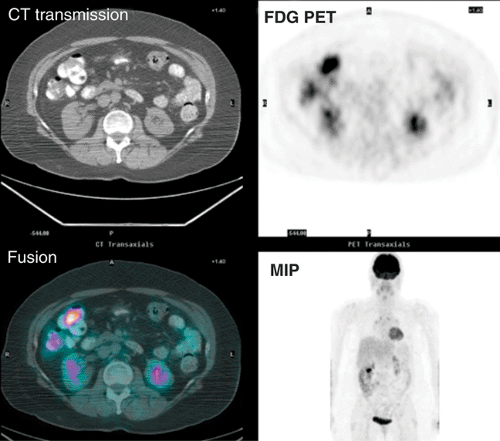
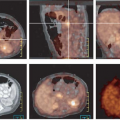
The clinical history, physical examination, pattern of uptake and correlation with anatomy are more helpful in avoiding false-positive interpretations than semiquantitative evaluation by the standard uptake value (SUV). The sensitivity of FDG PET is highly dependent on both the size of the lesion, reaching 72% sensitivity for lesions greater than 1 cm, and the degree of dysplasia reaching 89% for carcinomas, 76% for high-grade, and 36% for low-grade dysplasia (8). The sensitivity is more limited for flat premalignant lesions (9). Although PET is not recommended for detection or screening for precancerous or malignant colonic neoplasms, the identification of focal colon uptake should not be ignored. Fig. 8.14.1 illustrates incidental finding of colon carcinoma in a patient referred for FDG PET/CT follow-up of metastatic melanoma.
Initial Staging of Colorectal Cancer
The preoperative staging with imaging modalities is usually limited because most patients will benefit from colectomy to prevent intestinal obstruction and bleeding. The extent of the disease can be evaluated during surgery with excision of pericolonic and mesenteric lymph nodes along with peritoneal exploration. The performance of
FDG PET preoperatively may be helpful if the detection of distant metastases will cancel surgery in patients with increased surgical risk. It may also be helpful as a baseline evaluation prior to chemotherapy in patients with advanced stage disease. FDG PET imaging is a powerful tool for assessment of the response to therapy.
FDG PET preoperatively may be helpful if the detection of distant metastases will cancel surgery in patients with increased surgical risk. It may also be helpful as a baseline evaluation prior to chemotherapy in patients with advanced stage disease. FDG PET imaging is a powerful tool for assessment of the response to therapy.
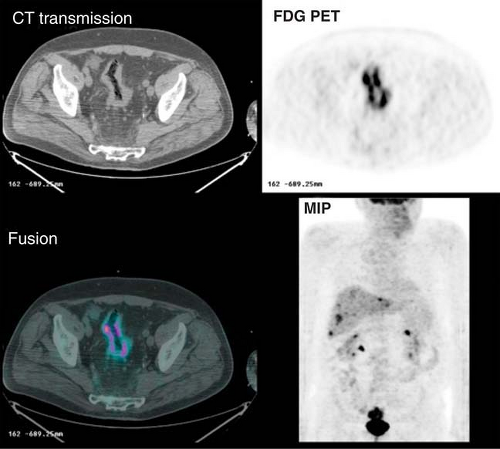
Three studies have been performed to evaluate the performance of FDG PET in the initial staging of colorectal cancer. Abdel-Nabi et al. (10) evaluated the usefulness of FDG PET for staging patients with known or suspected primary colorectal carcinomas. In 48 patients, FDG PET imaging identified all primary carcinomas. They found that both FDG and CT had a poor sensitivity of 29% for detecting local lymph node involvement. FDG PET was, however, superior to CT for detecting hepatic metastases, with sensitivity and specificity of 88% and 100%, respectively, compared to 38% and 97% for CT. These data were confirmed in the studies of Mukai et al. (11) and Kantorova et al. (12), which also reported that FDG PET changed the treatment modality in 8% of patients and the range of surgery in 13%. False-positive findings include abscesses, fistulas, diverticulitis, and occasionally adenomas. Although the sensitivity of FDG PET for the detection of a primary colon carcinoma may be high, its role in the preoperative staging is still debated except in high-risk patients for whom surgery can be avoided if metastases are identified, as illustrated in Fig. 8.14.2.
Detection of Recurrent or Metastatic Colorectal Carcinoma
The 2005 recommendations from the American Society of Clinical Oncology for colorectal cancer surveillance are: (a) history and physical examination every 3 to 6 months for the first 3 years, every
6 months during years 4 and 5, and subsequently at the discretion of the physician, (b) carcinoembryonic antigen (CEA) serum levels every 3 months postoperatively for at least 3 years after diagnosis, (c) annual CT of the chest and abdomen for 3 years after primary therapy for patients who are at higher risk of recurrence and who could be candidates for curative-intent surgery; pelvic CT scan for rectal cancer surveillance, (d) colonoscopy at 3 years after operative treatment, and, if results are normal, every 5 years thereafter (13).
6 months during years 4 and 5, and subsequently at the discretion of the physician, (b) carcinoembryonic antigen (CEA) serum levels every 3 months postoperatively for at least 3 years after diagnosis, (c) annual CT of the chest and abdomen for 3 years after primary therapy for patients who are at higher risk of recurrence and who could be candidates for curative-intent surgery; pelvic CT scan for rectal cancer surveillance, (d) colonoscopy at 3 years after operative treatment, and, if results are normal, every 5 years thereafter (13).
About 70% of the patients are resectable with curative intent but recurrence is noted in one third of these patients in the first 2 years after resection. Twenty-five percent of these patients have recurrence limited to one site and are potentially curable by surgical resection (14). For example, about 14,000 patients per year present with isolated liver metastases as their first recurrence, and about 20% of these patients die with metastases exclusively to the liver (15). Hepatic resection is the only curative therapy in these patients, but it is associated with a mortality of 2% to 7% and has the potential for significant morbidity (16). The poor prognosis of extrahepatic metastases is believed to be a contraindication to hepatic resection (17). Therefore, accurate noninvasive detection of inoperable disease with imaging modalities plays a pivotal role in selecting patients who would benefit from surgery.
Conventional Modalities for Detecting and Staging Recurrence
Elevated circulating levels of CEA occur in approximately two thirds of patients with colorectal carcinoma. Serial measurements of serum CEA levels are used to monitor these patients for recurrence and have a sensitivity of 59% and specificity of 84%. Imaging is necessary to localize the site of recurrence (18). Barium studies have been reported to detect local recurrence with an accuracy in the range of 80%, but are only 49% sensitive for overall recurrence; however, these are used relatively infrequently.
CT has been the conventional imaging modality used to localize recurrence with an accuracy of 25% to 73%, but it fails to demonstrate hepatic metastases in up to 7% of patients and underestimates the number of lobes involved in up to 33% of patients. In addition, metastases to the peritoneum, mesentery, and lymph nodes are commonly missed on CT, and the differentiation of postsurgical changes from local tumor recurrence is often equivocal (19,20,21,22,23). Among the patients with a negative CT, 50% will be found to have nonresectable lesions at the time of exploratory laparotomy. CT can also detect small lesions in the liver that are not due to malignancy resulting in false positives. CT portography (superior mesenteric arterial portography) is more sensitive (80% to 90%) than CT (70% to 80%) for detection of hepatic metastases, but has a considerable rate of false-positive findings, lowering the positive predictive value (24,25,26,27). In patients undergoing exploration for recurrent colorectal cancer, the presence of adhesions or the limitations of surgical exposure (transverse upper abdominal incision for liver resection) often preclude a detailed operative staging.
Detection and Staging Recurrent Colorectal Carcinoma with Fluorodeoxyglucose PET Imaging
A number of studies have demonstrated the role of FDG PET as a functional imaging modality for detecting recurrent or metastatic colorectal carcinoma (28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49). Overall, the sensitivity of FDG PET imaging is in the 90% range and the specificity greater than 70%, both superior to CT. A meta-analysis of 11 clinical reports and 577 patients determined that the sensitivity and specificity of FDG PET for detecting recurrent colorectal cancer were 97% and 76%, respectively (50). A comprehensive review of the PET literature (2,244 patients studied) has reported a weighted average for FDG PET sensitivity and specificity of 94% and 87%, respectively, compared to 79% and 73% for CT (51).
However, false-negative FDG PET findings have been reported with mucinous adenocarcinoma. Whiteford et al. (52) reported that the sensitivity of FDG PET imaging for detection of mucinous adenocarcinoma was 58% (n = 16), significantly lower than for nonmucinous adenocarcinoma at 92% (n = 93; P = .005). They suspect that the low sensitivity of FDG PET for detection of mucinous adenocarcinoma is due to the relative hypocellularity of these tumors. Similar findings (41% sensitivity) have been reported in a subsequent series of 22 patients (53).
Several studies have compared FDG PET and CT for differentiation of scar from local recurrence (29,30,33,34,35,39). CT was equivocal in most cases, and the accuracy of FDG PET imaging was greater than 90%. In the largest study (76 patients), the accuracy of FDG PET and CT were 95% and 65%, respectively (35).
Other studies have compared the accuracy of FDG PET and CT for detection of hepatic metastases (35,36,38,39,41). Overall, FDG PET was more accurate than CT. A meta-analysis performed to compare noninvasive imaging methods (ultrasound, CT, magnetic resonance imaging [MRI], and FDG PET) for the detection of hepatic metastases from colorectal, gastric, and esophageal cancers demonstrated that at an equivalent specificity of 85%, FDG PET had the highest sensitivity of 90% compared to 76% for MRI, 72% for CT, and 55% for ultrasound (54). A more recent meta-analysis confirmed these data (55). Vitola et al. (36) and Delbeke et al. (38) reported the comparison of FDG with CT and CT portography. CT portography, which is more invasive and more costly than FDG PET or CT alone, is regarded as the most effective means of determining resectability of hepatic metastasis by imaging. FDG PET had a higher accuracy (92%) than CT (78%) and CT portography (80%) for detection of hepatic metastases. Although the sensitivity of FDG PET (91%) was lower than that of CT portography (97%), the specificity of FDG PET was much higher, particularly at postsurgical sites.
Flanagan et al. (40) reported the use of FDG PET in 22 patients with unexplained elevation of serum CEA level after resection of colorectal carcinoma, and no abnormal findings on conventional work-up, including CT. Sensitivity and specificity of FDG PET for tumor recurrence were 100% and 71%, respectively. Valk et al. (41) reported sensitivity of 93% and specificity of 92% in a similar group of 18 patients. In both studies, PET correctly demonstrated tumor in two thirds of patients with rising CEA levels and negative CT scans.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree