- •
Exercise testing with MPI is preferred for evaluating symptomatic patients with known CAD to establish a link between symptoms and perfusion defects and for optimal risk stratification.
- •
Exercise capacity, duration of exercise, exercise-induced ST segment changes, or anginal symptoms are powerful prognostic markers obtained with exercise stress testing.
- •
In the absence of a large (>10%) fixed perfusion defect, patients with greater than 10% to 15% ischemia on SPECT appear to have a survival benefit with early revascularization compared with medical therapy alone.
- •
Low rest and a drop in poststress LVEF on radionuclide MPI are associated with increased cardiac risk.
- •
In cases of equivocal or inconclusive results on a SPECT MPI or in high-risk patients with known CAD, prior revascularization, or chronic kidney disease, PET MPI is preferred over SPECT if available.
- •
Radionuclide MPI can help quantify and localize ischemia as the etiology of new or worsening symptoms and help in management decisions.
- •
Transient ischemic dilation on PET is reflective of true stress-induced cavity dilation and is a high-risk imaging marker among patients with or without regional perfusion abnormalities.
- •
MBF assessment is less useful in patients with remote prior CABG because flows are often reduced from severe native vessel disease despite patent grafts.
- •
A decrease in regional peak hyperemic MBF and/or MBFR in a territory with or without a reversible perfusion defect is suggestive of single-vessel flow-limiting obstructive CAD.
- •
Threshold of inducible ischemia on PET above which benefit in survival and health status is noted with revascularization compared with medical therapy is lower compared with SPECT, especially when associated with globally reduced MBFR.
- •
In the absence of other high-risk markers or significant left main disease, it is reasonable to consider optimization of medical management initially among patients with significant ischemia (reversible perfusion defect >10%) noted on radionuclide MPI.
- •
Referral for revascularization can be considered in these patients if they remain symptomatic or have a very high burden of ischemia with other high-risk markers, such as reduction in stress LVEF or MBFR.
- •
PET is the preferred modality for evaluation of higher-risk patients with multiple comorbidities who are unable to exercise.
- •
Markers of high risk on a PET MPI study include the presence of large perfusion defects, transient cavity dilatation, a drop in LVEF with stress, and a severe reduction in MBFR throughout all coronary territories. These findings have all been associated with a markedly increased risk for adverse cardiac events, including cardiac death.
- •
In patients with multiple comorbidities who are at a high risk for left main or multivessel disease, PET should be considered over SPECT, because of availability of MBF information with PET.
- •
A reduced MBFR can be associated with significant multivessel obstructive CAD, diffuse disease, microvascular dysfunction, or a combination of all three processes. A patient with severely reduced MBFR (<1.6), especially if associated with other high-risk markers (e.g., low rest LVEF or a drop in LVEF, transient cavity dilatation) should always be considered for follow-up noninvasive or invasive coronary angiography.
- •
Regardless of the angiographic phenotype, a reduced MBFR (<2.0) is a marker of increased cardiovascular risk.
- •
MPI provides incremental risk stratification among patients with known CAD who are able to exercise and should be combined with exercise treadmill testing when feasible.
- •
In patients with a positive exercise ECG response and intermediate-risk Duke Treadmill score, a normal radionuclide MPI helps to reclassify them as low cardiac risk and can be managed medically.
- •
The low-risk warranty period of a normal perfusion SPECT among patients with known CAD is lower than among those without CAD, at approximately 2 years.
- •
Serial radionuclide MPI testing is considered rarely appropriate in asymptomatic patients with stable CAD within 2 years of initial diagnosis. Repeat testing is considered appropriate when the patient presents with new or worsening symptoms.
- •
Radionuclide MPI is considered appropriate for evaluation of obstructive CAD among patients with a CAC greater than 100, especially in the presence of symptoms.
- •
Risk for obstructive CAD rises with increasing CACS and it is higher if other cardiac risk factors are additionally present.
- •
Combined CACS and radionuclide MPI offers greater diagnostic accuracy in diagnosing obstructive CAD compared with MPI alone and improved risk stratification.
- •
The addition of MBFR by PET MPI improves confidence for ruling out significant obstructive CAD among patients with extensive CAC and normal MBFR and adds incremental risk stratification.
Introduction
Stable coronary artery disease (CAD) refers to all nonacute presentations potentially related to lack of adequate blood supply to the myocardium. Specifically, it excludes all of the acute coronary syndromes, such as unstable angina, ST elevation myocardial infarction (MI), and non-ST elevation MI. Although the time after which a patient with an acute presentation can be considered as having “chronic” or “stable” disease is somewhat arbitrary, patients who have gone a period of more than 6 months without new or recurrent symptoms after an acute event can be considered by guidelines as having known stable CAD. Included within the category of stable CAD are those with a prior diagnosis of stable angina and established diagnosis of obstructive or nonobstructive CAD on invasive or noninvasive coronary angiography, coronary artery calcium scan, or a stress test with or without imaging. It also includes patients with a past clinical history of acute coronary syndrome and previous revascularization for stable or acute CAD. The clinical manifestations of stable CAD are broad and include patients without symptoms, chronic symptoms of angina or angina-equivalents (predominantly dyspnea), new or worsening symptoms (chest pain, dyspnea, arrhythmias, syncope), or new signs of CAD (e.g., worsening left ventricle [LV] function, abnormalities on rest electrocardiogram [ECG]). This chapter will review the role of noninvasive testing for such patients, focusing mainly on myocardial perfusion imaging (MPI).
Role of non-invasive imaging in patients with stable coronary artery disease
In chronic stable CAD, noninvasive testing is usually triggered by a change in symptoms or signs suggesting that CAD may be progressing. The objectives of testing in those patients are to establish whether CAD is responsible for changes in symptoms or signs and to help with risk stratification and decisions regarding the possible need for revascularization. Multiple anatomic and functional noninvasive tests are now available. The critical first decision is whether to start with an anatomic evaluation or with functional testing. Coronary anatomy can be evaluated noninvasively with contrast-enhanced ECG-gated coronary computed tomography angiography (CCTA), whereas functional testing includes stress testing without imaging or associated with echocardiography, cardiac magnetic resonance imaging (CMR), and radionuclide MPI, including single photon emission computed tomography (SPECT) and positron emission tomography (PET). There are strengths and limitations to all of these approaches, and there is not one best choice for every patient presentation. Rather, a patient-centered approach should always be considered a best practice when choosing a noninvasive test for evaluation of patients with known stable CAD. The following text and Table 8.1 provide an overview of the major considerations surrounding test selection for patients with stable CAD.
- (1)
CCTA. CCTA offers the ability to noninvasively visualize obstructive CAD, quantify plaque burden, and identify high-risk plaque features. As discussed in Chapters 7 and 11 , it is a powerful tool for patients with suspected CAD but its role in patients with established CAD remains limited. One potential important role for CCTA in patients with known CAD is identification of those with left main disease, which may be missed by stress testing. In the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial, 5% of enrolled subjects with an ischemic functional test were found on CCTA to have unsuspected left main CAD. Nevertheless, there are many limitations to routine use of CCTA in patients with known CAD, including morbid obesity, high coronary artery calcium (CAC) scores, renal dysfunction, arrhythmias, and contrast allergies. In fact, CCTA is not recommended (Class III) for evaluation of patients with chronic CAD and severe coronary artery calcification because of reduced accuracy. Furthermore, the specificity and positive predictive value of CCTA are low in patients with stable CAD. , Obtaining information about the functional significance of stenosis is possible from CCTA using fractional flow reserve derived from computed tomography (FFR CT ) technology, which provides improved specificity and diagnostic accuracy compared with CCTA alone. Nevertheless, the low specificity of CCTA-based (42%) or FFR CT -directed (54%) assessment of stenosis severity can lead to unnecessary downstream invasive coronary angiography. FFR CT has similar low specificity (66%) in lesions of intermediate severity, where the role of noninvasive imaging modality in guiding posttest resource utilization would be most valuable. , Furthermore, FFR CT cannot be estimated accurately in patients with poor image quality, severe coronary calcification, or prior stents or coronary artery bypass grafting (CABG), which limits its use in most patients with known CAD. CCTA also has a low positive predictive value when assessing stent patency in patients post–percutaneous coronary intervention (PCI) because of limitations from artifact, especially in smaller stents. CCTA can be used to assess graft patency post-CABG; however, evaluation of distal anastomoses is often challenging because of motion and smaller vessel size, and assessment of native coronary vessels is limited because of extensive calcifications. Finally, an important parameter when evaluating patients with known CAD who are having new symptoms is global and regional left ventricular function. This is not attainable with CCTA without additional contrast and radiation and, therefore, is not routinely measured. Also, there is no data to support the use of CCTA for serial evaluation to evaluate progression in patients with already known CAD. For all of these reasons, CCTA is not commonly a test of first choice in this population.
- (2)
Exercise ECG testing. Functional assessment has been the mainstay for evaluation of patients with known CAD, and its use has been supported by society guidelines for many years. For patients with known CAD who are able to exercise to an adequate diagnostic workload and who have an interpretable ECG, standard exercise ECG testing remains a Class I indication. Exercise duration, ST segment changes, blood pressure response, symptoms, and arrhythmias are often sufficient to evaluate for whether or not symptoms are related to CAD, to differentiate levels of risk, and to decide if additional testing is advisable. Limitations include its inability to localize disease and quantify the extent and severity of ischemia, which is particularly important for patients with known CAD. Other limitations of ECG stress testing in patients with known CAD include the high prevalence of an abnormal baseline ECG and inability to complete diagnostic levels of exercise because of associated comorbidities, such as heart failure, atrial fibrillation, diabetes, and chronic kidney disease. Although exercise ECG testing is often used in low-risk patients without known CAD, it is less frequently a test of choice when used without imaging in patients with known CAD.
- (3)
Stress echocardiography. Stress echocardiography provides accurate assessments of regional and global LV function in response to exercise or dobutamine stress, offers high specificity among patients with known CAD, and can be used to assess myocardial viability as discussed in Chapter 20 . Although measurement of perfusion and myocardial flow is possible with vasodilator stress echocardiography, it is technically challenging and not commonly used in practice. Patients with known CAD often have baseline wall motion and/or conduction abnormalities, which can lower the accuracy of this test. Sensitivity of stress echocardiography for patients with known CAD is lower compared with other functional imaging modalities.
- (4)
Stress CMR. Stress CMR is performed with vasodilator or dobutamine stress, can provide perfusion information along with regional wall and global LV wall motion, and can offer a viability assessment. It offers high sensitivity and specificity among patients with known CAD without the risks of ionizing radiation. Although not routinely available, it also offers the possibility to quantify tissue perfusion and measure myocardial blood flow (MBF). Use of stress CMR is limited in those with coexisting chronic kidney disease because of use of gadolinium contrast; those with pacemakers/defibrillators, which are frequently incompatible or can cause image artifacts; and those who are unable to cooperate with breath hold or are claustrophobic. Functional imaging with MPI mitigates those limitations with stress CMR and has extensive data supporting its use among patients with known chronic CAD, which will be discussed in detail in this chapter.
| Radionuclide MPI | CCTA | Stress CMR | Stress Echocardiography | |
|---|---|---|---|---|
| Technical limitations | Attenuation artifacts with SPECT, corrected with use of attenuation correction; less common with PET | Heavy calcification; stents can lead to a “blooming” artifact and nondiagnostic test | Accuracy reduced in patients with an irregular heart rate and cardiac devices | Views can be suboptimal based on patient habitus; high rate of false positives with left bundle branch block and significant baseline wall motion abnormalities |
| Viability assessment | Yes, with use of F-18 FDG tracer | No | Yes, with late gadolinium enhancement | Yes, with use of low-dose dobutamine stress |
| Test duration | 2–4 hours for SPECT, 15–40 minutes for PET | 5–10 minutes | 30–45 minutes, including viability | 30 minutes |
| Contrast use | No | Yes, iodinated | Yes, gadolinium based | +/− ultrasound enhancing agents |
| Caution in chronic kidney disease | No | Yes | Yes, contraindicated in eGFR <30 mL/min/m 2 | No |
| Ionizing radiation exposure | Yes | Yes | No | No |
| Claustrophobia | D-SPECT and Anger SPECT cameras can be used | Avoid | Avoid | Most suitable |
Radionuclide MPI in patients with known coronary artery disease
In complex high-risk patients with stable CAD, including those with prior MI, prior revascularization, arrhythmias, and heart failure, radionuclide MPI is the most commonly used noninvasive imaging test. An extensive literature supports its use to determine whether new or changing symptoms or signs (e.g., worsening LV function) reflect CAD progression; for risk stratification; and for patient management, especially to help with decisions regarding the possible need for revascularization. Radionuclide MPI provides accurate quantification of ischemic and scar burden. Importantly, it can be performed safely in virtually any patient independent of body habitus and weight, with or without arrhythmias, and in all stages of kidney failure. In addition, it is not limited by the ability of the patient to hold a breath and can be performed in patients with conduction abnormalities and pacemakers or defibrillators. Its use in conjunction with vasodilator stress is particularly useful and safe in patients with suspected unstable symptoms. This is especially important in patients with known CAD who present with new or worsening symptoms that may or may not represent unstable angina. It is, however, associated with exposure to ionizing radiation, which can accumulate with multiple procedures, especially in patients with known CAD. Potential approaches to minimize radiation exposure are discussed in greater detail in Chapters 4 and 6 . The choice of radionuclide MPI modality (SPECT vs. PET), imaging protocol and type of stressor (exercise vs. pharmacologic) should be tailored to patient characteristics, risk factors and comorbidities, clinical questions, and local availability. As discussed in Chapter 4 , combined rest-stress or stress-rest 1- or 2-day protocols are recommended. In select patients with elevated calcium scores where SPECT MPI is performed for detection of functionally significant stenoses in affected areas, stress-only protocols may be used. The specific approaches to the patient with known CAD, prior MI, and severe LV systolic dysfunction are discussed in Chapter 20 .
In patients with prior CABG, radionuclide MPI is helpful in assessing patency of bypass grafts. This is a relatively simple task because there is already detailed knowledge of coronary anatomy and the placement of grafts. Regions of ischemia may identify nonrevascularized portions of myocardium, failed grafts, anastomotic disease, compromised side-branches, or the development of new disease. In patients who have had prior stenting, MPI is useful for identifying stent closure, jailing of side-branches, or new disease. Again, knowledge of prior anatomy facilitates the localization of abnormalities to specific coronaries and etiologies of ischemia. In patients with known total coronary artery occlusions, radionuclide MPI provides accurate quantitative assessments of ischemia and scar to help with decision making.
Differences between SPECT and PET myocardial perfusion imaging
Most radionuclide MPI studies around the world are performed with SPECT. Radiotracers and protocols for SPECT and PET are discussed in Chapter 4 . Major differences between SPECT and PET are discussed in the following sections and summarized in Table 8.2 .
| SPECT | PET | |
|---|---|---|
| Type of acquisition | Sequential | Simultaneous list mode acquisition |
| Commonly used tracers | 99m Tc sestamibi, 99m Tc tetrafosmin, 201 thallium | 13 N ammonia, 82 Rb, 18 F flurpiridaz, 15 O water |
| Photon energy (keV) | 68–167 | 511 |
| Radiation | ∼ 7–15 mSv | ∼ 3–4 mSv |
| Spatial Resolution | 12–15 mm | 4–6 mm |
| Attenuation and Scatter Correction | Uncommon | Routine use in all studies |
| Timing of Stress Imaging | 30–60 minutes after vasodilator stress, 15–30 minutes after exercise stress | Peak stress |
| Measurement of absolute myocardial blood flow values | Possible, research ongoing | Yes, routine |
| Type of stressor used | Exercise or pharmacologic | Pharmacologic only; exercise possible with 13 N-ammonia and 18 F-flurpiridaz |
| Diagnostic Accuracy | Less robust compared with PET | Best among all modalities on head-to-head comparison |
| Prognostic Ability | Very good | Excellent, added value of LVEF reserve and myocardial blood flow reserve |
| Ability to measure coronary microvascular dysfunction | No (tested in research environment) | Yes |
Assessment of regional myocardial perfusion
Regional myocardial perfusion with SPECT and PET MPI is assessed using a semiquantitative visual analysis of the rest and stress imaging using a 17-segment model. The total perfusion deficit at rest (summed rest score) and stress (summed stress score) is then calculated by adding the individual scores from all 17 LV segments and expressed as a percent of the LV mass. Ischemia (reflecting the extent of reversibility of perfusion deficit from stress to rest) and scar (reflecting the extent of fixed perfusion defect) burden are then calculated as the difference between the total stress and rest score and also expressed as a percent of the LV mass. It is important to note that this assessment of myocardial perfusion is spatially relative; that is, the myocardial segment with the best perfusion is assumed to be normal. Consequently, the spatially relative quantification of myocardial perfusion may be misleadingly normal or mildly abnormal in patients with diffuse coronary stenoses, causing a balanced reduction in MBF (e.g., significant left main or multivessel CAD). Likewise, a lacking or attenuated response to vasodilator stress can have a similar effect in such patients.
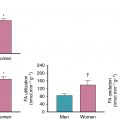
A major limitation of SPECT is that of attenuation of radiotracer uptake from soft tissues surrounding the heart (such as breast or diaphragm), which can affect the interpretation and estimation of ischemia and scar burden (see Chapters 1 and 5 ). Correction of soft-tissue attenuation or combined supine and prone imaging can help mitigate this problem and improve diagnostic accuracy. In women, breast tissue and implants can result in apparent reductions in tracer uptake in the anterior wall, suggesting a lesion in the left anterior descending artery (LAD) territory. Given that LAD territory ischemia represents high potential risk, such findings can result in referrals to coronary angiography. Likewise, in men with abdominal obesity, there can arise apparent perfusion defects that affect the inferior portions of the heart.
Unlike SPECT, PET MPI offers increased spatial and contrast resolution, high signal-to-noise ratio because of higher photon energy of PET radiotracers, and the use of routine and robust attenuation correction for all images (see Chapter 2 ). As such, PET image quality is less affected by patient characteristics such as gender, body habitus, and size, resulting in improved sensitivity, specificity, and overall diagnostic accuracy compared with SPECT. Perhaps the most valuable advantage of PET over SPECT is its ability to routinely measure absolute MBF at rest and during maximal stress and get an estimate of the myocardial blood flow reserve (MBFR; the ratio of stress over rest MBF). This complements the spatially relative perfusion assessment previously described and improves delineation of myocardium at risk. This single measure of tissue perfusion captures the extent and severity of myocardial ischemia resulting from single or serial focal stenotic lesions, diffuse atherosclerotic disease, and/or microvascular dysfunction compared with focal epicardial stenoses alone. The utility of myocardial flow assessments among patients with known CAD is discussed in detail in a case-based approach later in this chapter.
Assessment of left ventricular function
As described in Chapter 4 , LV function is routinely quantified using ECG-gated data at rest and stress. Because the stress SPECT images are usually acquired beginning approximately 10 to 15 minutes after peak exercise stress or 45 to 60 minutes post–peak vasodilator stress, the stress LV ejection fraction (LVEF) assessment with SPECT is a poststress measurement and not at true peak stress LVEF. In contrast, PET offers the ability to acquire stress images at or near peak stress. Consequently, the change in LVEF from rest to peak stress is more directly associated with myocardial ischemia in patients undergoing PET and is an important complement to the perfusion assessment for diagnosis and risk stratification. ,
The timing of acquisition of SPECT and PET images also has implications on the pathophysiologic significance of transient ischemic dilation with stress (calculated as poststress summed LV volume/rest summed LV volume). The presence of transient ischemic dilatation on SPECT images is thought to be related to diffuse subendocardial hypo-perfusion causing an increase in the apparent size of the LV endocardial cavity, whereas on PET images, it is generally related to true stress-induced cavity dilation and transiently enlarged LV volumes. Regardless of the mechanism, the presence of transient cavity dilatation is a marker of higher clinical risk.
Patient radiation dose
As discussed in Chapters 4 and 6 , PET MPI is associated with consistently lower radiation doses to patients.
Prognostic markers from radionuclide MPI
Table 8.3 summarizes all the prognostic markers available from the SPECT and PET MPI and used to guide patient management, which will be discussed in the section on patient-centered applications of radionuclide MPI. In addition, please see the discussion in Chapter 17 .
| Low Risk (<1% annual risk of death/MI) a | Intermediate Risk (1%–3% annual risk of death/MI) | High Risk (>3% annual risk of death/MI) |
|---|---|---|
| Low-risk treadmill score ($5), no new ST-segment changes or exercise-induced chest pain symptoms at maximal levels of exercise | ≥1 mm of ST segment depression occurring with exertional symptoms | ≥2 mm of ST-segment depression at low workload or persisting into recovery, exercise-induced ST-segment elevation, or exercise-induced ventricular tachycardia |
| Inability to exercise, necessitating use of pharmacologic stress | Impaired heart rate reserve ≤4 bpm | |
| Calcium Score <100 | Calcium Score 100–399 | Calcium Score ≥400 |
| Rest LVEF ≥50% | Rest LVEF 35%–49% | Rest LVEF ≤35% |
| Normal or small myocardial perfusion defect at rest or with stress, affecting <5% of the LV mass | Resting perfusion defect involving 5%–9.9% of the LV mass in patients without a prior MI | Resting perfusion defect ≥10% of the LV mass in patients without prior MI |
| Stress-induced perfusion defect involving 5%–9.9% of the LV mass or defect in one vascular territory without LV dilation with stress b | Stress-induced perfusion defects involving ≥10% myocardium or defects in multiple vascular territories | |
| Normal stress or no change of limited resting wall motion abnormalities during stress | Small wall motion abnormality involving one to two segments and only one coronary territory PET myocardial blood flow reserve 1.5–2.0 | Inducible wall motion abnormality (involving more than two segments or two coronary territories) Severe stress-induced LV dysfunction (peak stress LVEF <45% or drop in LVEF with stress ≥10% on SPECT or PET LVEF Reserve <0) Transient ischemic dilation >1.13 c PET myocardial blood flow reserve <1.5 Increased RV tracer uptake Increased lung uptake |
a All findings suggestive of low-risk if present in absence of any other higher-risk findings on MPI.
b In absence of myocardial blood flow reserve <2.0 for patients undergoing PET.
c In association with one or more other high-risk features for SPECT.
Patient-centered applications of radionuclide MPI in patients with known coronary artery disease: Case-based discussion
Case vignette 1: New or worsening symptoms in a patient with stable angina who is able to exercise
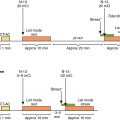
A 59-year-old man with a history of dyslipidemia, smoking, and stable angina and a family history of premature CAD was diagnosed with CAD 5 years prior based on symptoms and ST changes on a treadmill exercise test. He presented to the office with a 3-month history of exertional chest discomfort. He was compliant with his antianginal regimen. An exercise myocardial perfusion SPECT was ordered because of new symptoms despite optimal medical management. He exercised for 9.3 minutes of a modified Bruce protocol, achieving 10.1 metabolic equivalent tasks (METs) of activity, with a normal heart rate and blood pressure response. Exercise was terminated because of chest pain and shortness of breath. His resting 12-lead ECG showed sinus rhythm. After 3 minutes and 50 seconds of exercise, there was 1 mm of horizontal ST-segment depression in inferior leads. At peak exercise, there was approximately 2 mm of horizontal ST-segment depression in inferior and lateral leads, which persisted through the 4 minutes of recovery. His Duke Treadmill Score was −8.7 (intermediate risk).
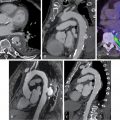
The SPECT images ( Fig. 8.1 ) showed a large, severe, and mostly reversible perfusion defect anteriorly, anteroseptally, and apically, consistent with a large area of severe ischemia mixed with some scar in the distribution of the LAD coronary artery. Quantitatively, 21% of the LV was ischemic and 5% infarcted. The gated images showed mildly depressed resting LVEF of 49% with mild global hypokinesis. There was a fall in LVEF poststress to 47%, with worsening regional wall motion in the LAD territory. The coronary artery calcium scoring (CACS) was estimated to be 228. The patient underwent a subsequent coronary angiogram, which showed ostial chronic total occlusion of the LAD coronary artery. The patient subsequently underwent successful PCI of his LAD chronic total occlusion 2 months later because of persistent symptoms despite medical therapy optimization.
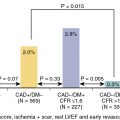
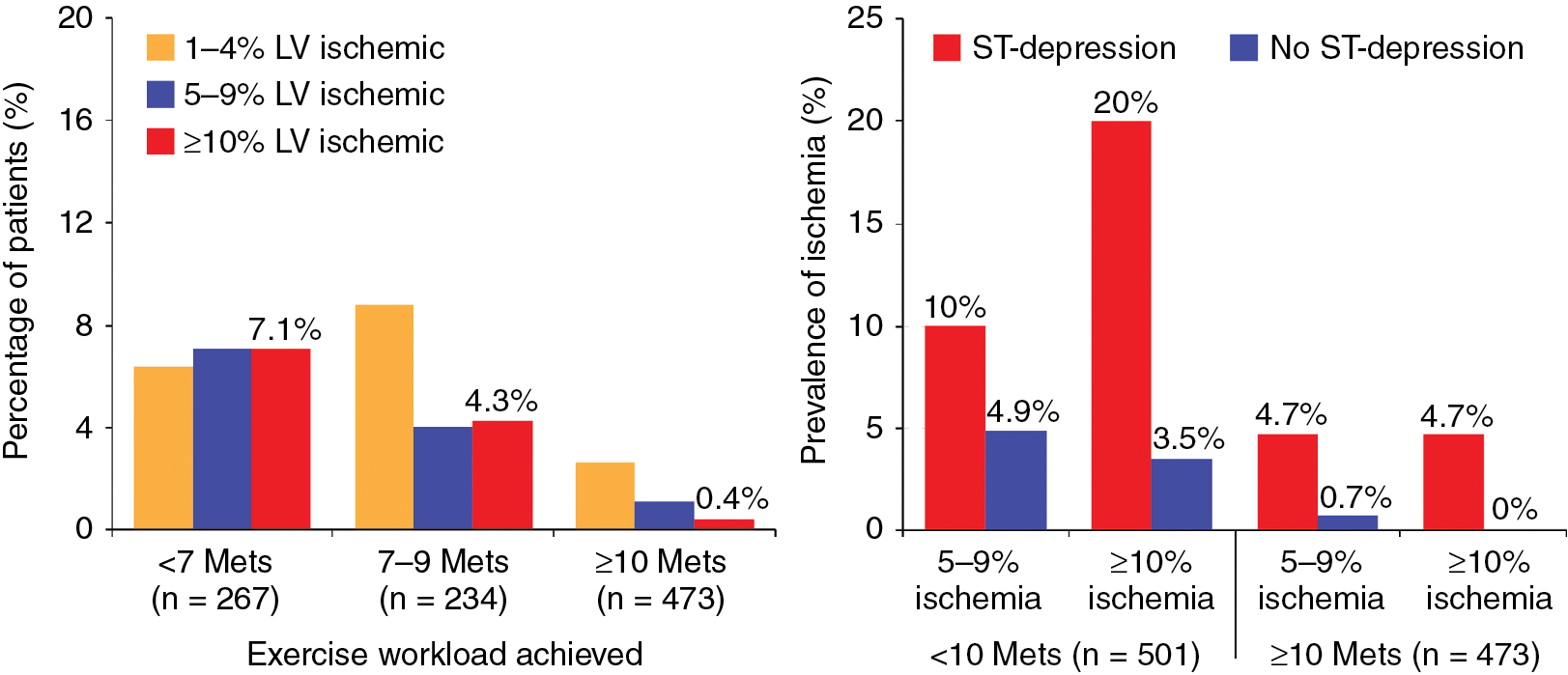
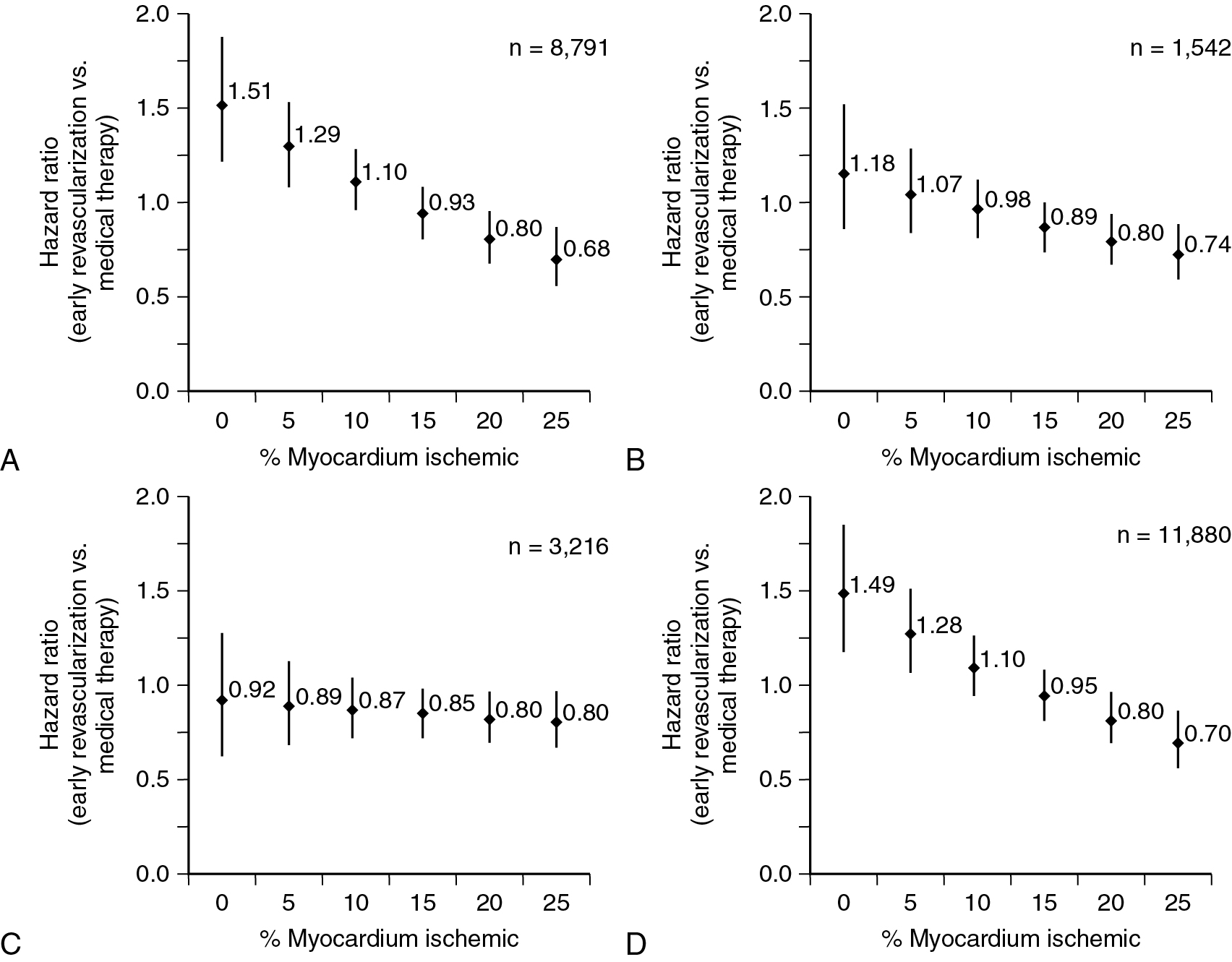
Among patients with known CAD able to perform exercise, exercise MPI is preferred because it provides valuable complementary diagnostic and prognostic information to the imaging data, including exercise capacity, heart rate and blood pressure response, and arrhythmias. In patients with an established diagnosis of CAD, combined exercise ECG testing with MPI is recommended because the clinical question is not about diagnosing CAD but rather localizing and quantifying the ischemia burden and guiding specific treatment strategies. Exercise also helps reproduce the patient’s symptoms and, importantly, confirms the symptoms are the result of CAD and linked to the scan findings, as illustrated in Case Vignette 1. Nevertheless, vasodilator stress is recommended for patients with left bundle branch block or paced rhythm or those who are not able to achieve a reasonable exercise workload. , Patients who are able to exercise 10 METs or more rarely have such large amounts of ischemia as did this patient. , Patients with ischemic ST-segment depression have a higher risk for having inducible ischemia despite achieving a higher workload of exercise ( Fig. 8.2 ). Failure of recovery of ST-segment changes to baseline postexercise is another high-risk marker. Patients with anginal symptoms with exercise have threefold to fourfold higher incidence of cardiac events compared with patients with ST-segment depression, and thus this is another high-risk marker on the test. This patient also had multiple high-risk markers on his SPECT MPI, including a large perfusion defect with stress involving 26% of the LV mass, most of which was reversible, indicating extensive and severe ischemia and was associated with a drop in LVEF postexercise, which helped with further risk stratification. , , Patients without prior MI and less than 10% scar on their SPECT MPI associated with greater than 12% to 15% inducible ischemia, like in Case Vignette 1, appear to have a survival benefit with early revascularization compared with medical therapy only ( Fig. 8.3 ). Finally, the presence of significant inducible ischemia in a territory supplied by a chronic total occlusion helps support clinical decision making, especially in light of the fact that PCI is associated with a higher risk for complications. Thus, in patients with known CAD, exercise testing can provide important prognostic information. Combining SPECT imaging with exercise testing provides additional risk stratification and helps with localization of disease and posttest management decisions, even in patients with good functional capacity.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree