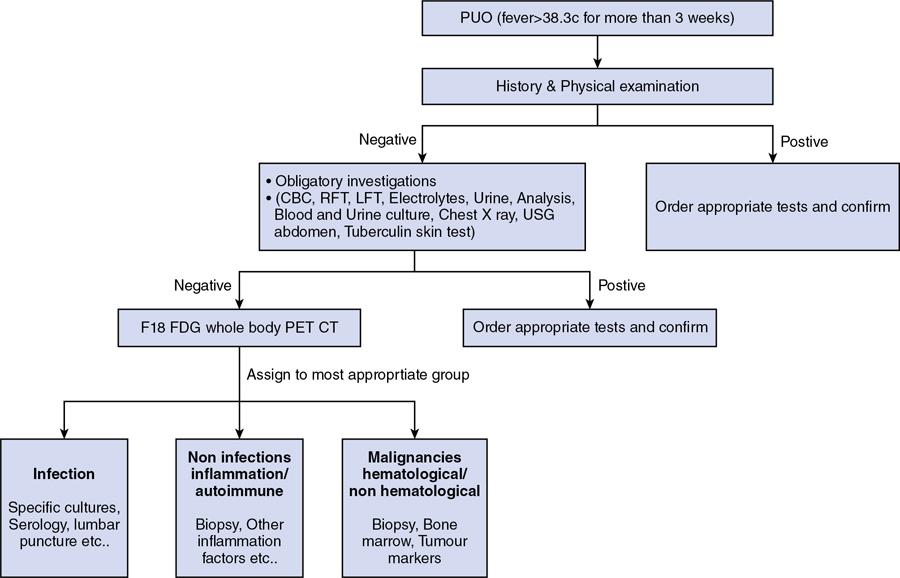
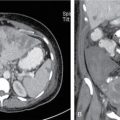
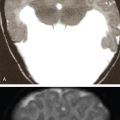
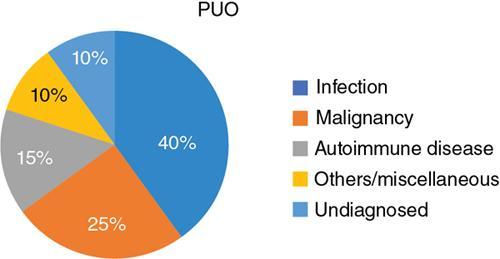
L Murali Krishna, Vigneshwaran, Gurubharath Pyrexia of unknown origin (PUO) is described as a condition with body temperature greater than 38.3°C (100.9°F) for more than 3 weeks with an unknown source inspite of all relevant investigations. The four subgroups of the differential diagnosis of PUO are infections, malignancies, autoimmune conditions and miscellaneous. Setting the period at ≥3 weeks was to exclude self-limited viral infections, which usually resolve in under 3 weeks. A thorough history, physical examination and standard laboratory testing remain the basis of the initial evaluation of the patient with PUO. With the availability of several diagnostic modalities such as serology, viral cultures, CT and MRI, there is a significant change in the assessment of patients with PUO. The term PUO is, however, being more loosely used by clinicians for any patient who presents with fever of unknown source after performing very limited investigations. A typical request form for a CT chest/abdomen in the radiology department would read – “? PUO, for evaluation”. More recently, the term inflammation of unknown origin has been coined for cases of unexplained chronic inflammation without fever. Unsurprisingly, there is a certain degree of overlap between the causes of pyrexia/fever of unknown origin and inflammation of unknown origin. Usual method of approaching the patient with pyrexia of unknown origin (Table 2.12.1). Though by definition PUO suggests fever of unknown origin, the pyrexia’s have a pathophysiological basis. Based on the pathology, the patients can be classified in four categories: (1) infections, (2) malignancies, (3) noninfectious inflammatory diseases or (4) miscellaneous disorders including drug-related fever, hyperthermia and factitious fever (Fig. 2.12.1). The phrase “noninfectious inflammatory diseases” is used for a number of conditions such as rheumatic diseases, autoimmune diseases, systemic diseases, collagen vascular diseases or vasculitis, connective tissue diseases and granulomatous diseases. In the recent past, the cases of PUO caused due to infection and neoplasm have significantly reduced because of the diagnosis of solid masses and lymph nodes by ultrasound and computed tomography (CT). Almost 50% of the patients with PUO have to undergo extensive noninvasive and invasive testing before a provisional diagnosis can be reached. In the extensive literature that exists on PUO, there is no diagnostic gold standard against which other diagnostic tests may be measured. Because of this, we have several algorithms to approach a patient with PUO and it varies from region to region. A series from India published in 2001 showed that infection featured more prominently than in Western series with tuberculosis, bacterial endocarditis and abscesses all accounting for a significant share of cases. However, noninfective causes were broad and varied including cancers and inflammatory disorders. Malignancies were both haematological (Hodgkin and non-Hodgkin lymphoma) and visceral (colon cancer, ovarian carcinoma and bronchogenic carcinoma). Noninfectious inflammatory diseases included lupus, Takayasu arteritis, mixed connective tissue disease, ankylosing spondylitis and polyarteritis nodosa. Other causes in this series, each diagnosed in a single patient, included sarcoidosis, granulomatous hepatitis, autoimmune hepatitis, atrial myxoma and a drug fever. Although the diagnostic approach in PUO has not been uniform, the critical step is to elicit a medical history and a through physical examination of the patient. A complete and repeated medical history is important, including information about alcohol intake, medications, occupational exposures, pets, travel, familial disorders and previous illnesses. In the past, fever curves were reported to be useful as few specific patterns correlated with certain causes. However, several studies have correlated that the pattern, peak and duration of fever do not lead to any specific diagnosis. Noninvasive investigations such as fundoscopy of eye and other investigations such as bone marrow aspiration and bronchoalveolar lavage can have better specificity but are hampered by their relatively low negative predictive value. This limitation is essential and may be overcome with the use of imaging techniques, especially F18-FDG PET/CT seems to be very promising. Imaging techniques such as radiographs, ultrasonography (US), computerized tomography (CT), magnetic resonance imaging (MRI) are used to access the anatomy while functional imaging modalities include planar/total body scintigraphy and single photon computerized photography (SPECT) and positron emission tomography (PET). F18-FDG PET/CT has an evolving role in PUO, and to understand the same, we need to be aware of the advantages and disadvantages of the other modalities. Plain-film radiography: Chest radiography is useful for diagnosis intrathoracic and intraabdominal pathologies. Elevated hemidiaphragm or pleural effusion with atelectasis is seen in patients with subphrenic, splenic, hepatic and pancreatic abscesses. Sometimes intraabdominal masses are seen in the subdiaphragmatic part of the radiograph. Radiograph can show typical findings of soft-tissue swelling, although they may not be apparent during the early phases of disease. For an osseous lesion to appear on a radiograph, it might take 2–3 weeks, and by this time, there is significant loss of bone density. Ultrasonography: USG is widely available, quick, inexpensive and not associated with radiation exposure. But the results are highly operator-dependent. Also many deep structures of the body cannot be visualized by USG due to the limited penetration of the sound waves through gas and bone. Echocardiography may help to diagnose infective endocarditis in demonstrating vegetations; however, sometimes the vegetations may not be infective and other times they may not be seen clearly due to presence of prosthetic valves. The transesophageal approach has a greater sensitivity of almost 92%, while the transthoracic approach has a sensitivity of 60%. Moreover, ultrasound can be used in the diagnosis of other conditions such as myxoma, sarcoidosis and other infections. Lower limb Doppler has helped in the diagnosis of deep vein thrombosis which can be the cause of PUO in few patients. It is a simple and safe procedure and can be performed in patients with PUO. Computerized tomography: CT is highly reproducible, has an excellent spatial resolution, is relatively inexpensive and has very short scanning time, usually less than 10 min. The examination time is short, generally less than 5–10 min. The only limitation is the high radiation because of which it cannot be repeated in frequent intervals. Moreover, contrast cannot be administered for patients with renal impairment and those with allergic reactions. CT provides only anatomical data but lacks functional information of the imaged organs. False-negative CT has been reported primarily because of some distortion in anatomy of microabscesses or due to nonuse of contrast agents. In neutropenic patients with PUO, pneumonia can be diagnosed early by using low-dose multislice CT, which has low radiation burden. Magnetic resonance imaging: MRI has very high spatial resolution, which provides excellent structural resolution. MRI has zero radiation exposure and gives some functional information as well. The disadvantages are the long scanning times resulting in artefacts, limitation to scanning patients with implants and also relatively expensive. MRI is very useful in diagnosing pathologies of the bone marrow, muscles, tendons, ligaments, cartilage and small organs such as the prostate gland, testes, cervix and uterus. Whole-body MRI may be able to provide better anatomical differentiation than the CT imaging without radiation burden. However, the nonspecific natures of the findings and longer imaging time make it less preferable investigation. In patients to whom radiation is to be avoided, such as pregnancy and younger patients, whole-body MRI is useful as tool of choice. Radiological investigations greatly depend on structural alterations, and it becomes difficult to diagnose malignant, infectious or inflammatory foci in the early phase because of the lack of substantial anatomical changes at that time. The anatomy also gets altered postsurgery or radiotherapy, and it becomes difficult to differentiate infectious or inflammatory lesions from residual anatomical changes. The functional and metabolic imaging especially with scintigraphy methods further enhance the diagnostic process of patients with PUO. The evolution of hybrid PET/CT was a significant milestone in medical imaging. Hybrid PET/CT accelerates the diagnosis of PUO patients because of the availability of both anatomical and metabolic information. The high spatial resolution also helps to localize and characterize tissues with increased metabolic activity, and thereby, the process of finding a pathological diagnosis is easier. The most widely used PET tracer is the glucose analogue 2-deoxy-2-(F-18) fluoro-d-glucose (F18-FDG). In oncology patients, there are increased glycolytic rate in malignant cells and overexpression of glucose transporters (GLUT-1 and GLUT-3). Intracellularly, FDG is phosphorylated to FDG-6-phosphate by hexokinase; however, since FDG-6-phosphate is not a suitable substrate for the glycolytic enzymes that follow, FDG-6-phosphate continues to accumulate intracellularly. Similarly, inflammatory cells and granulation tissue also use glucose as its energy source, and there is increased accumulation of F18-FDG detected by PET. The pathologies commonly diagnosed with the help of F18 FDG PET/CT can be classified into infection, inflammatory, malignancy and autoimmune disease. PET/CT has a sensitivity of more than 90% in diagnosing infections. Active tuberculosis, focal abscesses, atypical pneumonias, focal nephritis and colitis are some of the infections that can be picked up early in PET/CT when other investigations are normal. Infective endocarditis is another common infection that can be picked up early using PET/CT. Infected vascular grafts or orthopaedic prosthesis can also be diagnosed by PET/CT. Tuberculosis, however, remains the most common cause for infection in the developing countries (Figs 2.12.2–2.12.5).
2.12: Approach to a case of pyrexia of unknown origin – radiology and imaging perspective
Introduction
Diagnosis of pyrexia of unknown origin
Early clinical assessment
Imaging available to examine the patient with pyrexia of unknown origin
F18-FDG positron emission tomography/computerized tomography
Infectious conditions
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree