This article describes an approach to analyzing the distribution of intravenous contrast on chest computed tomography and illustrates the various pathologies and pitfalls that may be encountered by the imager, especially in the hospitalized patient. Understanding normal and abnormal distribution of intravenous contrast can be used as a clue to detect alterations in physiology and flow.
Key points
- •
Abnormal distribution of intravenous contrast may be a clue to a compromised cardiovascular system in hospitalized patients.
- •
Inadequate transit of contrast or incomplete opacification of structures, such as the left ventricle or aorta, normally expected to be opacified, should prompt the imager to search for a potential cause, such as myocardial infarction or cardiac tamponade.
- •
Use of delayed venous-phase imaging is a strategy that can be used to reduce the occurrence of flow artifacts.
Introduction
Hospitalized patients frequently undergo contrast enhanced computed tomography (CT) examinations for a variety of clinical indications. This patient population is also uniquely predisposed to both important to recognize pathologies as a result of hemodynamic instability and vascular intervention as well as mimics of pathology resulting from their underlying disease state.
Often, hospitalized or ICU patients have impaired or altered cardiovascular function, and these findings often manifest on CT as alterations in blood flow—therefore, alterations in contrast distribution or flow. For example, decrease in myocardial contractility frequently manifests on contrast enhanced CT, because this imaging modality is dependent on adequate systolic function to homogeneously perfuse the imaged vessels and tissues. Abnormal contrast enhancement, however, not only is a symptom of the many pathologies that hospitalized patients may face but also can be useful diagnostically and prognostically to identify unsuspected disease or complications.
When abnormal contrast enhancement is present, knowledge of the underlying physiology can help the interpreting radiologist confidently distinguish artifacts from true pathology, improving the accuracy of interpretation of contrast enhanced chest CT in critically ill patients.
This article provides an approach to the assessment of contrast distribution on contrast-enhanced chest CT and then reviews several specific situations encountered when imaging hospitalized patients, including evaluation of patients with systolic dysfunction, interpretation of heterogeneous flow, and mimics of enhancement, such as calcification and embolized materials.
Systolic dysfunction
Reflux of Contrast into the Inferior Vena Cava, Cardiogenic Shock, and Asystole
Reflux of contrast into the inferior vena cava (IVC) (assuming injection is performed via the upper extremities) is a common phenomenon on arterial-phase CT and does not necessarily indicate the presence of severe cardiac dysfunction when seen in isolation. In distinction, a true blood-contrast level in the IVC is a rare but often emergent imaging finding that signifies impending cardiovascular collapse. A discussion of each of these appearances follows.
The presence of intravenous (IV) contrast in the IVC is a potential imaging biomarker for right heart disease and should prompt the imager to look for further clues to the presence of pathology. Knowledge of the route and rate of injection of contrast is important in this evaluation because rate of injection can affect the degree of reflux and injection from a lower extremity approach is going to make this assessment impossible.
Many early case reports or small series described the association of IVC reflux with various right-sided cardiac pathologies, including pulmonary hypertension, tricuspid regurgitation, atrial fibrillation, and right ventricular systolic failure. Yeh and colleagues were the first group to perform a large retrospective review (127 patients) and found that reflux of contrast into the IVC or hepatic veins was significantly associated with right heart disease, including tricuspid regurgitation, pulmonary hypertension, or right ventricular systolic dysfunction. The investigators reported, however, a sensitivity of only 31%. Importantly, they found that at higher injection rates typical of CT angiography (CTA) studies (>3 mL/s), the specificity of IVC reflux for one of these pathologies was only 69%, whereas when the rate of injection was slower (less than <3 mL/s) the specificity was 98%.
More recently, Aviram and colleagues retrospectively reviewed 967 CT pulmonary angiogram (CTPA) studies and semiquantitatively graded the severity of reflux on a scale of 1 to 6 (1, no IVC reflux; 2, trace reflux only in the IVC; 3, reflux into the IVC but not the hepatic veins; 4, reflux into the IVC and proximal hepatic veins; 5, reflux into the IVC and midpart of the hepatic veins; and 6, reflux into the IVC and distal hepatic veins). They found that extensive reflux (grades 4–6) was significantly associated with pulmonary hypertension (odds ratio [OR] 5.4), congestive heart failure (OR 3.7), chronic atrial fibrillation (OR 2.3), and acute pulmonary embolism (OR 1.8).
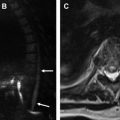
Although several studies have shown that reflux of contrast into the IVC can be seen in the setting of acute pulmonary embolism, the prognostic utility of this finding remains uncertain. Bach and colleagues reviewed 365 CTPA studies of acute pulmonary embolism and found that reflux into the hepatic veins (grades 4–6, as described previously) was an independent predictor of 30-day mortality (OR 3.29). More recently, however, Beenen and colleagues reviewed 1950 patients with acute pulmonary embolism (PE) and found no significant correlation between presence of IVC contrast reflux and either short-term (1-week) or long-term (1-year) mortality ( Fig. 1 ).

Cardiogenic shock and, rarely, asystole/cardiac arrest represent the most severe forms of cardiac dysfunction in critically ill patients, and patients in these physiologic states occasionally may be imaged on CT. Cardiogenic shock is defined as a state of severely reduced cardiac output despite adequate intravascular volume whereas asystole represents the complete cessation of cardiac function. There are numerous causes, including acute myocardial infarction (MI), acute decompensation of heart failure (eg, dilated cardiomyopathy and myocarditis), dynamic outflow tract obstruction, cardiac tamponade, pulmonary embolism, and sepsis.
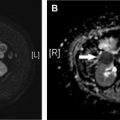
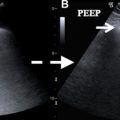
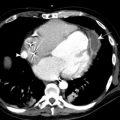
The main imaging finding in patients imaged with severely depressed cardiac output is pooling and dependent layering of contrast. Many terms have been used to describe this phenomenon, including dependent pooling, blood-contrast level, and the IVC level sign, and all explain the concept that severely reduced (or, in the case of asystole, absent) cardiac output means that there is no force to combat gravity. Hence, the contrast settles in the vena cava and other dependent structures like the right lobe of the liver, the kidneys, and the lumbar venous plexus, because it is heavier than blood. Furthermore, little contrast is propelled forward into the pulmonary arteries and may be entirely absent on the systemic side of the circulatory system. The authors have also observed contrast layering in the left atrium after passing through a patent foramen ovale (thus bypassing the pulmonary circulation) ( Figs. 2 and 3 ).


A blood-contrast level is an emergent finding. Cardiogenic shock carries a short-term mortality of up to 80%, if not recognized, and patients with asystole require immediate resuscitation.
Less severe levels of systolic dysfunction can also lead to slow contrast transit through the heart, which may manifest as increased enhancement of the right-sided heart chambers with poor opacification of the left heart and aorta. Chaturvedi and colleagues measured differential enhancement of the aorta and main pulmonary artery in 137 emergency department patients with suspected but absent pulmonary embolism and identified that those with poor left ventricular [LV] function (LV ejection fraction <40%) had several distinct features—aortic to pulmonary artery enhancement ratio of 0.2 to 1 had a sensitivity of 54% and specificity on 93% for LV systolic dysfunction. Not unexpectedly, those with poor LV function also had a more prolonged contrast transit time on bolus timing sequences. The investigators concluded that unsuspected LV systolic dysfunction can be identified on pulmonary embolism CT, and this realization may be important for the appropriate triage and management of patients presenting with otherwise uncharacterized cardiovascular complaints in the emergency department.
Cardiac Tamponade
Patients with cardiac tamponade also present with symptoms and imaging findings compatible with reduced cardiac output. Cardiac tamponade is the hemodynamic state of reduced cardiac filling that results from accumulation of any substance in the pericardial sac (usually fluid, such as blood) that increases pericardial pressure and restricts cardiac chamber filling. Because the fibrous pericardium is relatively noncompliant, even amounts as small as 100 mL to 200 mL can cause tamponade in the acute setting. Because tamponade is a potentially life-threatening condition, imagers need to be aware of the imaging appearance and search for any explainable cause.
When a pericardial effusion is present (especially if it is large), then the imager should look for signs of tamponade. Indirect CT findings that suggest elevated filling pressures include enlargement of the superior vena cava greater than the diameter of the thoracic aorta, distention of the IVC, reflux of contrast into the azygos vein or IVC, dependent pooling of contrast, and enlargement of the hepatic veins. Flattening of the right heart chambers (the flattened heart sign), leftward bowing of the interventricular septum, and compression of intrapericardial structures like the pulmonary trunk or coronary sinus are more direct findings that can increase confidence in the diagnosis. Causes of tamponade are numerous and include hemopericardium, other pericardial fluid such as malignant effusion or pericarditis, and rarely air resulting in tension pneumopericardium.
Acute Myocardial Infarction on Computed Tomography
Finally, acute decline in cardiac function as the result of MI also may be identified with CT. Patients with a high suspicion of acute coronary syndrome should not undergo chest CT as a first-line diagnostic modality. However, 20% to 60% of patients experiencing an acute MI may have atypical cardiopulmonary symptoms (eg, atypical chest pain and dyspnea) or abdominal symptoms (eg, nausea and vomiting) that prompt CT evaluation. Thus it is imperative that imagers recognize the findings of myocardial ischemia/MI on any contrast-enhanced CT that includes the heart, especially when no other readily identifiable causes for the symptoms are present on the CT.
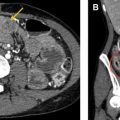
Acute MI is recognizable as an area of decreased LV myocardial enhancement that corresponds to a vascular territory. The decreased attenuation may involve just the subendocardial layer or may be transmural depending on the severity of the infarct. Even on older-generation CT scanners, the findings of acute MI may be apparent—Gosalia and colleagues retrospectively reviewed 18 patients who underwent single-slice CT and found that a region of relative hypoattenuation of the LV in a vascular territory (decrease of more than 20 Hounsfield units [HU] relative to normal myocardium) was 83% sensitive and 95% specific, with positive predictive value of 94% and negative predictive value of 86% for diagnosis of acute MI. Other studies have shown similar results with specificities and sensitivities greater than 80% on newer generation CT scanners both for arterial-phase CT angiography and for parenchymal-phase 100-second delay CT. Depending on the amount of myocardium involved, patients experiencing an acute MI may have findings of pulmonary edema, reduced transit of contrast, and/or reflux of contrast into the IVC (as discussed previously) ( Fig. 4 ).

Beam hardening, respiratory motion, and cardiac motion are common artifacts that may be mistaken for MI, especially in the posterobasal LV wall. Especially when there is question of the reality of the finding, the imager should discuss with the clinician the possibility of MI and prompt further evaluation with electrocardiogram and or serum biomarkers.
Heterogeneous flow
In general, the imager should not assume that contrast enhancement is uniform throughout a vessel. Nonuniform contrast enhancement of the thoracic vasculature on contrast-enhanced chest CT and CTA may result from a variety of physiologic and pathologic processes. Modern CT scanners have the benefits of better temporal resolution and shorter scan times; however, they are also more prone to flow-related artifacts. These artifacts can lead to misdiagnosis by mimicking pathology but, however, can also be illustrative of subtle cardiovascular abnormalities, such as shunts. The authors have recently described the principles of this phenomenon, termed smoke , in a recent publication. In general, smoke is seen when there is fast temporal resolution and when there is inadequate time for contrast to mix (due to a combination of reduced cardiac output or other cause of reduced contrast transit, mixing of opacified and unopacified blood, and/or scanning at the leading edge of the bolus). Although detailed management strategies for these problems are beyond the scope of this article, scanning with a longer delay can allow more time for mixing to occur as described later.
Pulmonary Embolism
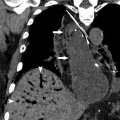
The basic principles needed to correctly distinguish flow artifact from intravascular filling defect due to thrombus include a firm grasp on the imaging appearance of thrombus. True thrombus appears as a discrete filling defect with defined margins and a density in the range of 30 HU to 60 HU. The mean attenuation of acute pulmonary embolism has been investigated and reported at 33 HU ± 15 HU, with greater than 99% of pulmonary embolism falling below 100 HU in density. Contrast mixing, on the other hand, appears ill defined and has a wide range of attenuation based on the contrast density, however, often exceeding 100 HU. Contrast mixing mimicking pulmonary embolism (which may be termed smoke based on its wispy, ill-defined appearance) can be seen in a variety of clinical settings, including (1) scanning very early at the initial edge of the contrast bolus; (2) regional alterations in pulmonary arterial blood flow as a result of vascular stenosis, vasoconstriction, or down-regulated flow in areas of chronic parenchymal disease; and (3) venous or collateral inflow ( Fig. 5 ).