Chapter 6 APPROACH TO BONE AND SOFT TISSUE TUMORS AND TUMOR-LIKE CONDITIONS
Evaluating bone and soft tissue tumors requires a multi-modality approach. Each modality has advantages and disadvantages, and a rational, tailored algorithm should be used. Reflexive, simultaneous ordering of all modalities when a lesion is found or clinically suspected should be discouraged.
MODALITIES
Ultrasound
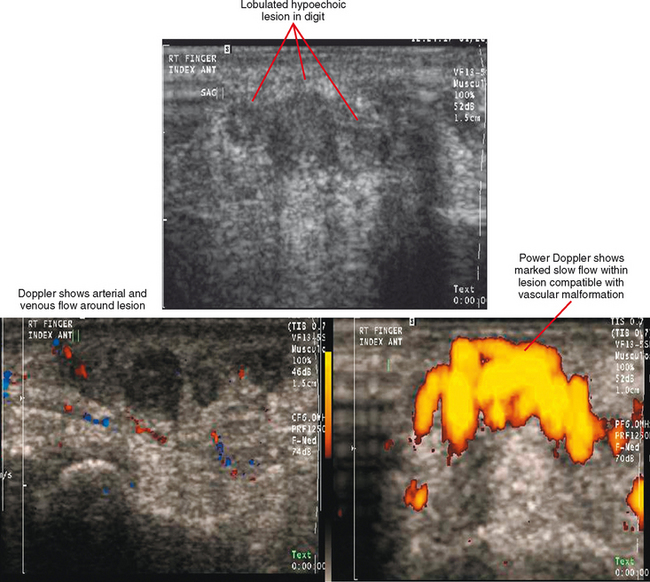
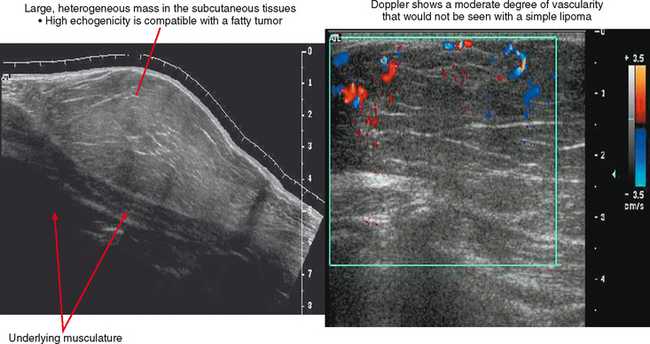
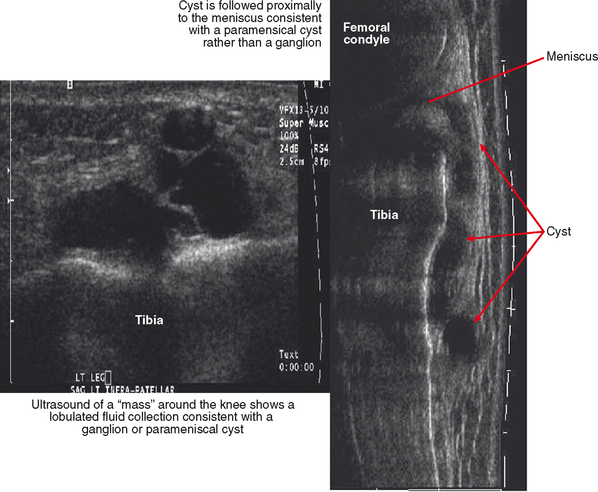
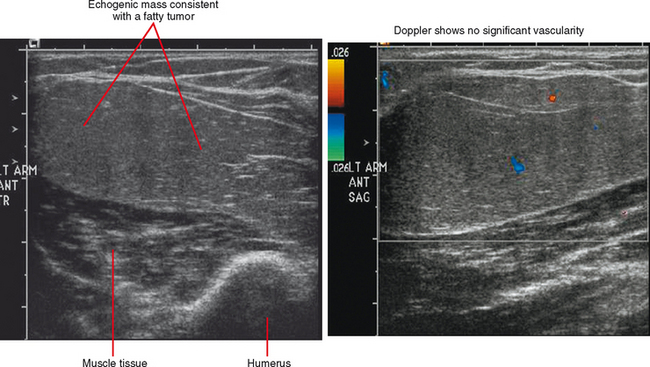
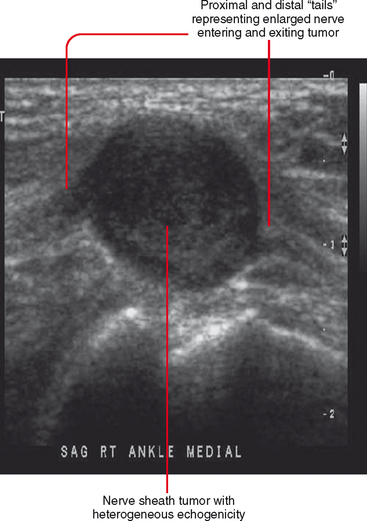
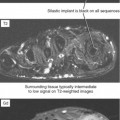
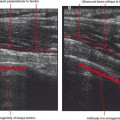
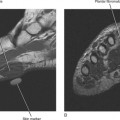
Ultrasound is valuable in differentiating cystic from solid soft tissue lesions and has also been used to study vascularity of lesions (Figs. 6-1 and 6-2). For soft tissue prominence at a joint, ultrasound may offer a specific diagnosis (e.g., ganglion cyst and paralabral or parameniscal cyst; Fig. 6-3). However, ultrasound is not as useful for characterizing pathology or defining the extent of true soft tissue masses, except in situations in which lesion echogenicity (e.g., lipoma; Fig. 6-4) or morphology (e.g., nerve sheath tumor, Fig. 6-5) is specific.
Magnetic Resonance Imaging
When routine radiographic features of a bone lesion are indeterminate or when the lesion is more aggressive and considered to be potentially malignant, MRI is frequently required and is useful for characterization as well as staging. Soft tissue masses always require evaluation with CT or MRI. More commonly MRI is used unless there is calcification that CT could better characterize. Gadolinium contrast is useful for distinguishing cystic, myxoid, or necrotic components from solid regions; it is also useful when scanned in dynamic fashion rapidly after a bolus to characterize tumor vascularity. Although certain features on MRI can often be used to narrow the differential diagnosis for bone and soft tissue, it is unreliable for characterizing tissue type and is not even specific for distinguishing many benign from malignant lesions. Therefore, the radiologist should not become overconfident and should always keep in mind the limitations of each modality as well as the broad range of nontumors and tumor-like lesions that can simulate true neoplasms.
Nuclear Medicine
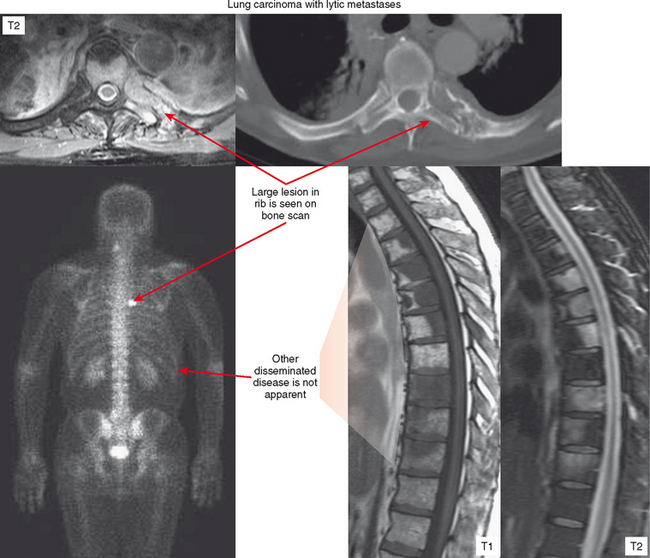
Bone scan is predominantly useful for evaluation of multiplicity of lesions. However, if the primary lesion in question (or lesion detected with another modality) shows no increased uptake on bone scan, the scan should be considered of limited value for detecting other deposits. This is often the case with purely lytic metastases and myeloma (Fig. 6-6). On the other end of the spectrum, diffuse involvement (skeletal carcinomatosis), as seen in cases of metastatic prostate or breast carcinoma, can produce such generalized uptake that the bone scan looks relatively normal except for lack of uptake by the kidneys (described as a “superscan”). Bone scan is also very useful for detecting the metastatic lesions of osteosarcoma, which avidly concentrate radiotracer. Osteosarcoma tends to metastasize to lung, and bone scan can be helpful for surveillance of recurrence as well as distant metastases.
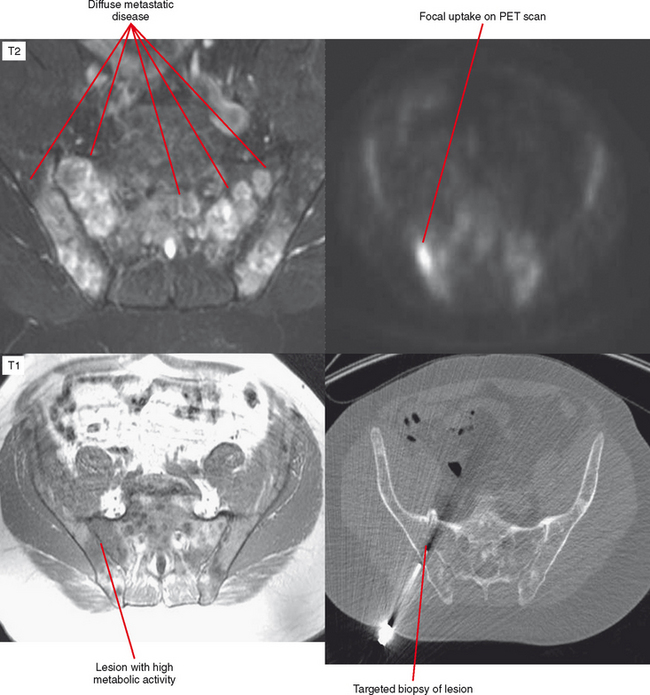
Another situation in which bone scan can be useful is the differentiation of a benign sclerotic focus, such as a bone island or fibrous dysplasia with little to no increased uptake, from a blastic metastasis, which has a high degree of uptake. For patients with ill-defined symptoms and normal radiographs, a radionuclide bone scan may be used to localize the abnormality. Following a positive bone scan, either MRI or CT may be selected to better define the nature of the lesion. Radionuclide studies are not indicated in most situations for evaluation of soft tissue masses. Techniques such as positron-emission tomography (PET) scanning have been used mainly for evaluation of metastatic disease and follow-up of treated lesions but show promise for identifying active areas of a tumor before biopsy (Fig. 6-7).
TUMOR-LIKE LESIONS (BONE OR SOFT TISSUE) ON IMAGING EXAMINATIONS
GENERAL TIPS: BONE OR SOFT TISSUE TUMORS
Appearance of Lesions Recently Biopsied
Biopsy makes a lesion look more aggressive on CT and MRI, resulting in surrounding edema and enhancement that makes it difficult or impossible to define the lesion’s true margins. If a biopsy is near a neurovascular bundle, this can change a planned surgery from a limb salvage to an amputation. It is very important to obtain all imaging before biopsy.
Appearance of Treated Lesions
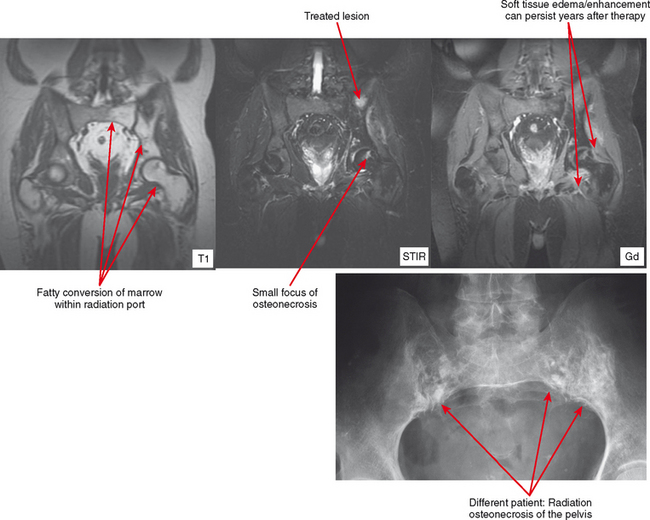
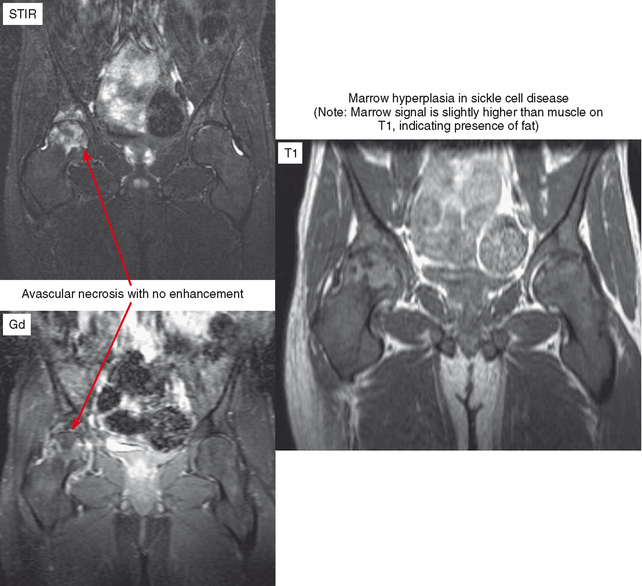
The appearance of bone and soft tissue can change after treatment. Considering the lesions themselves, lytic, metastatic lesions can become more sclerotic, especially breast metastases. Bone scan may demonstrate a paradoxic increase in activity because of bone healing (flare phenomenon). The pattern of red marrow can change on MRI. For example, rebound following chemotherapy or treatment with colony-stimulating factor can result in an increase in hematopoietic marrow, which is often more cellular and thereby brighter in T2 and STIR signal than typical red marrow. Radiation therapy results in replacement of red marrow with fatty yellow marrow (Fig. 6-8). Treatment can also affect the soft tissues. For example, radiation therapy causes soft tissue edema, especially of the underlying muscles within the port. This edema may last for years and can simulate a number of pathologic conditions from trauma to infection to neurologic disease; no mass effect is seen. In later follow-up, the muscles may exhibit fatty atrophy.
Staging of Primary Musculoskeletal Tumors
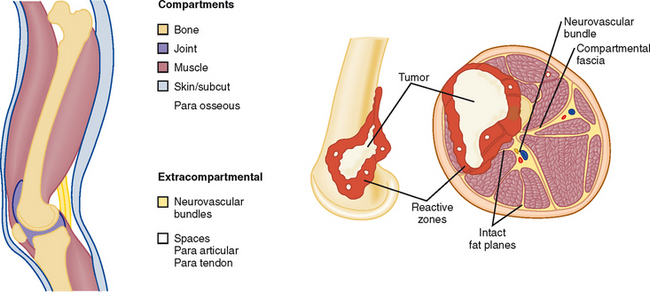
Description of the imaging appearance of a bone or soft tissue tumor should include a description of local aggressiveness and extension into other compartments, such as from bone to adjacent muscles, from one fascial compartment to another, from anywhere into a neurovascular compartment, and distant metastases (Fig. 6-9 and Table 6-1). This information is combined with histologic aggressiveness (Grade 0: G0 = benign; Grade 1: G1 = low-grade malignant; Grade 2: G2 = high-grade malignant) to determine the overall stage (Table 6-2).
Table 6-1 Anatomic Compartments
| Intracompartmental (A) | Extracompartmental (B) |
|---|---|
| Intraosseous | Soft tissue extension |
| Intra-articular | Soft tissue extension |
| Superficial to deep fascia | Deep fascial extension |
| Paraosseous | Intraosseous or extrafascial |
From Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop 1980; 153:106–120.
Table 6-2 Surgical Stages of Sarcomas
| Stage | Grade | Site |
|---|---|---|
| 1A | Low (G1) | Intracompartmental (T1) |
| IB | Low (G1) | Extracompartmental (T2) |
| IIA | High (G2) | Intracompartmental (T1) |
| IIB | High (G2) | Extracompartmental (T2) |
| III | Any (G) | Any (T) |
| Regional or distant metastasis |
From Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcomata. Clin Orthop 1980; 153:106–120.
GENERAL APPROACH TO BONE TUMORS
PITFALLS AND PSEUDOTUMORS
Stress Fracture
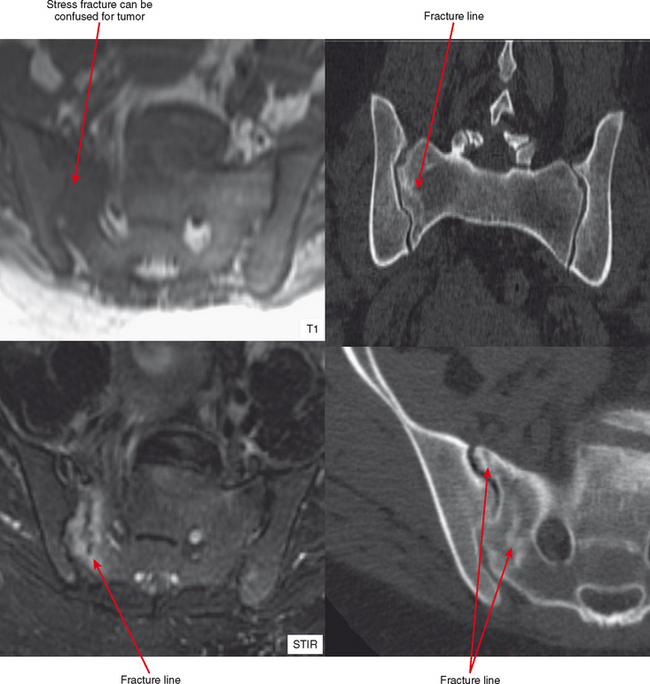
It is not common for stress fractures to be confused with tumor, since the clinical context usually guides the referring doctor to request specifically to “rule out stress fracture.” Yet it continues to occur, as evidenced by fractures referred for biopsy. There are clearly situations in which the radiologist is more likely to confuse stress fracture with tumor. Biopsy of such a lesion can have devastating results, as in an erroneous pathologic diagnosis of osteosarcoma (due to mitoses and osteoid formation), so it is important to be aware of the following situations.
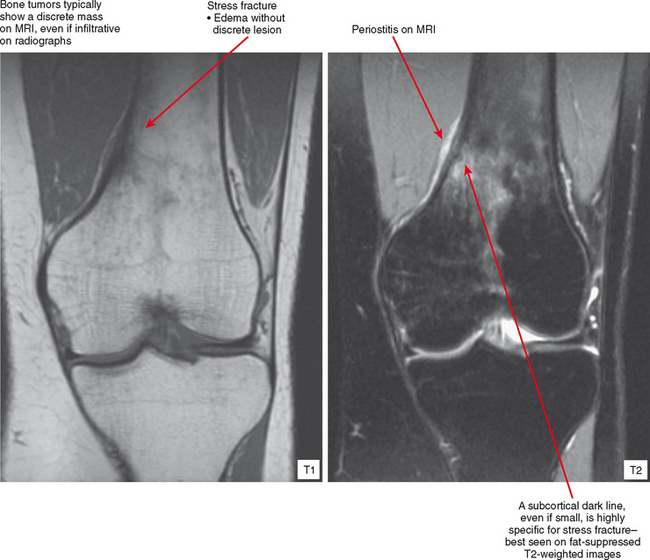
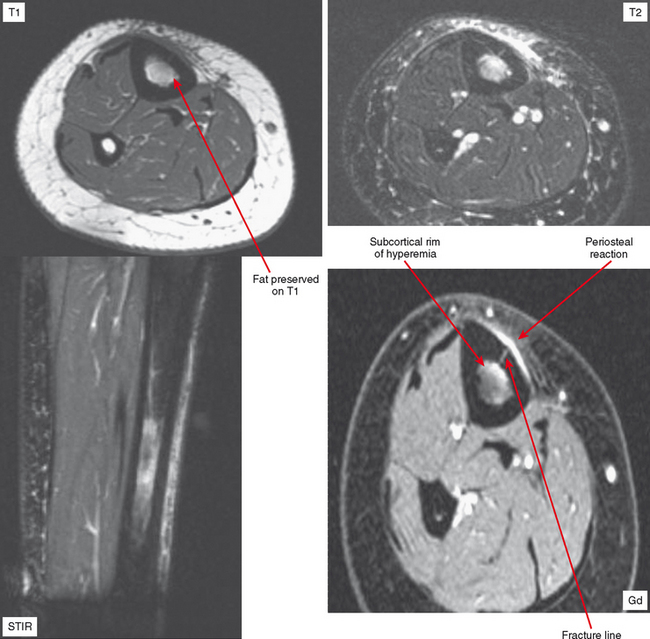
Radiographs and CT may early reveal indeterminate or confusing findings, such as subtle periosteal reaction or an area of bone sclerosis. Given the clinical context, look closely for sclerosis to be primarily subcortical in location, with the fracture oriented perpendicular to the trabecular pattern. Bone scan shows a focus of markedly increased uptake, usually eccentric, but the pattern is nonspecific. MRI can confirm a suspected diagnosis, with a characteristic dark line on T1- and T2-weighted images extending from the cortex into the medullary cavity, surrounded by edema (see Fig. 6-12). Tumors, on the other hand, show a discrete lesion on MRI without the dark line.
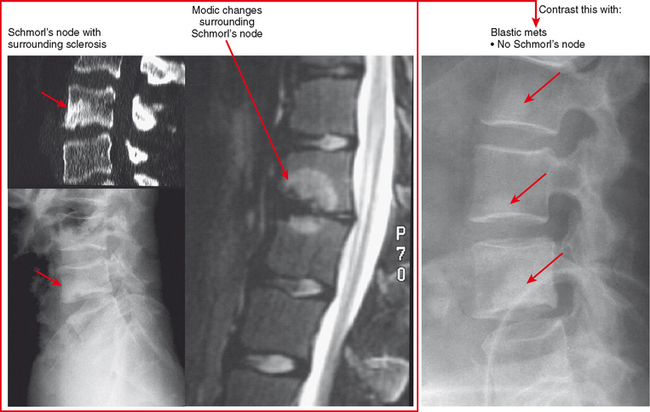
Schmorl’s Node and Hemispheric Spondylosclerosis
Schmorl’s nodes are often associated with reactive sclerosis, or hemispheric spondylosclerosis. When extensive, this can simulate a sclerotic metastasis (Fig. 6-13). On radiographs, communication with the adjacent disk and associated degeneration may not be apparent, but this can be confirmed with CT or MRI.
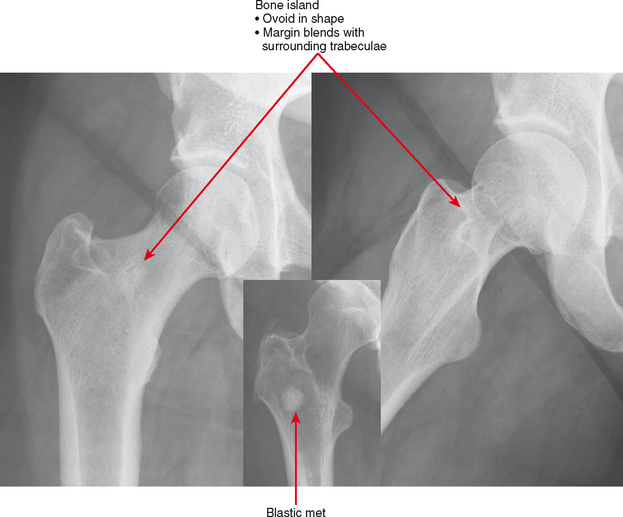
Bone Island (Enostosis) Versus Blastic Tumor
Bone islands are essentially benign hamartomas consisting of a focus of cortical bone within medullary bone. They can simulate a solitary metastasis on radiographs. Bone islands are usually seen as solitary or scattered foci but are seen in greater numbers in tuberous sclerosis and sclerosing bone dysplasias. There are a few imaging features suggesting their benign etiology. First, the margins of bone islands classically “blend” with the adjacent trabeculae. Also, bone islands tend to be ovoid in shape; metastases tend not to be round (Fig. 6-14). These characteristics are not as comforting when reviewing an examination of a patient with a history of cancer. In this setting, a bone scan is very useful. Bone islands show minimal to no increased uptake, whereas, if large enough, a blastic metastasis should show intense uptake. In addition, bone scan can detect other lesions. CT may detect the trabecular blending better than bone scan, but it is not usually very useful. MRI can be useful because bone islands are typically monotonous black signal on all sequences, whereas metastatic lesions often contain some T2 hyperintensity and enhancement, even if blastic. PET scan could be useful, again, only if the lesion is large enough to detect. Like bone scan, if the PET scan is negative and the lesion is less than 1 cm, further workup (such as biopsy) may still be necessary.
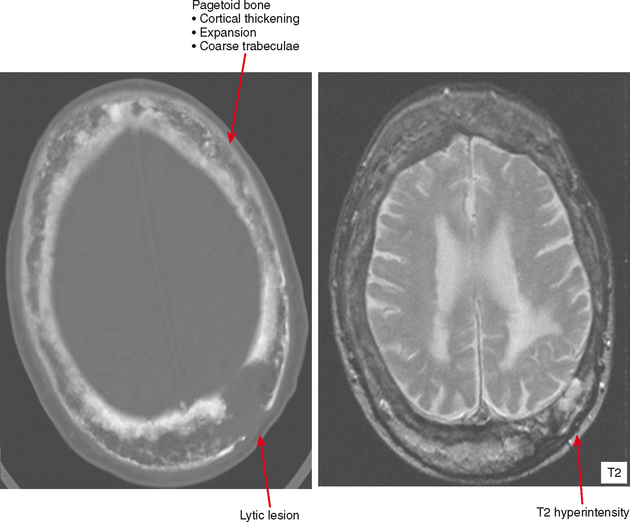
Paget Disease
In the healing, or reparative, phase, bone is laid down appositionally (i.e., along the cortex) and within the medullary cavity, causing the bone to appear denser and forming the characteristic hallmarks of Paget disease: cortical thickening, trabecular coarsening, and an appearance of bone expansion. In its softened state, the deformities of Paget disease occur in affected bones owing to stresses from weight-bearing. This includes protrusio acetabuli and bowing of long bones, with a “shepherd’s crook deformity” of the proximal femur. Deformity at joints can cause osteoarthritis, and expansion of bone can result in narrowing of vascular and neural foramina.
Late involvement of Paget disease is characteristic on radiographs, with the hallmarks as above. However, in unclear cases, CT is useful to confirm the presence of cortical thickening and fat density in the marrow. Bone scan is quite specific, with geographic uptake as previously discussed. MRI often shows some fat in the underlying marrow. Secondary tumors can occur, and any focal lytic areas, cortical destruction, or soft tissue mass associated with pagetoid bone should be pursued (Fig. 6-15). Sarcomas, including osteosarcomas, chondrosarcomas, and fibrosarcomas can occur and are very aggressive with a poor prognosis. Giant cell tumors can also occur and appear very similar, but usually without a significant soft tissue mass. Biopsy is needed to document histology of suspicious areas.
Sclerosing Bone Dysplasias
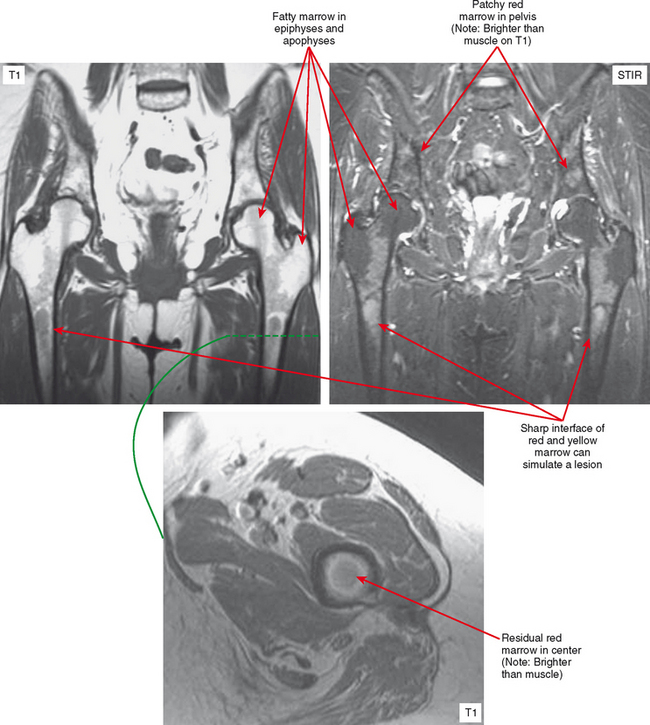
Osteopoikilosis, osteopathia striata, and melorheostosis are sclerosing bone dysplasias, which are benign conditions resulting in areas of bone formation. This dysplasia occurs either within the medullary cavity, as with osteopoikilosis and osteopathia striata, or along the cortex and adjacent soft tissues, as with melorheostosis. Osteopoikilosis is the one most often confused with tumor. In this condition, numerous small foci of sclerotic bone representing bone islands are seen in the epiphyses and apophyses. The correct diagnosis is usually suggested by the fact that this distribution is the opposite of typical metastatic disease, which usually spares the growth centers. Osteopathia striata (Voorhaave disease) has a linear pattern of medullary sclerosis and is not usually confused with malignancy. In melorheostosis (Fig. 6-16), the bone formation is along the cortex and/or adjacent soft tissues (dripping wax pattern) in a sclerotomal distribution along the neural supply of the affected bones. Because of this, multiple bones may be involved along the nerve distribution. These dysplasias can also appear together in mixed patterns.
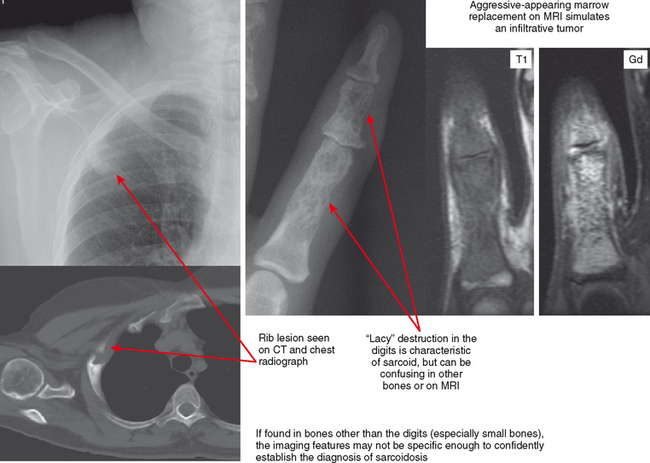
Sarcoidosis
Sarcoid is most common in the middle and distal phalanges of the hands and feet but can involve any bone. The classic lacy destruction and lack of periosteal reaction are usually recognized in the phalanges, but in other bones the pattern may mimic an aggressive neoplasm. The bone lesions are usually painful. When seen in young adults, these lacy lesions should prompt correlation with history or chest radiographs. If imaged with MRI alone, the findings may not appear characteristic, and the lesion can be misinterpreted as an aggressive one (Fig. 6-17).
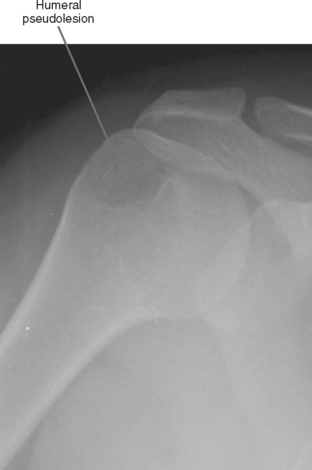
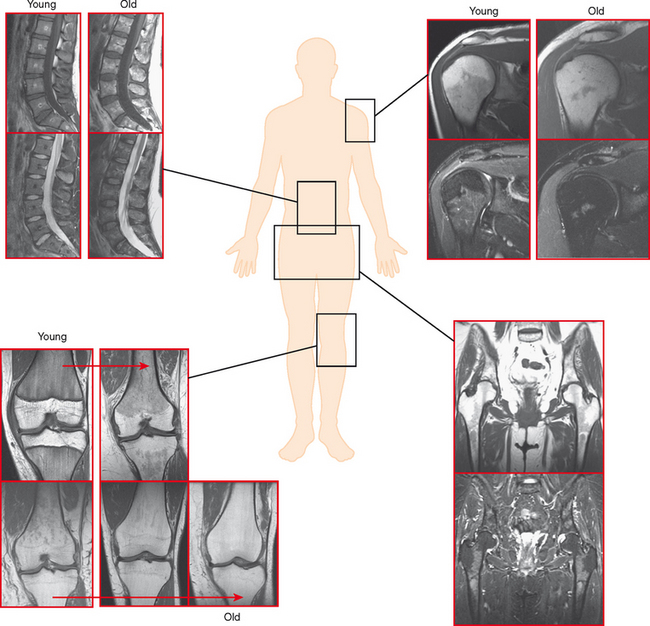
Pseudolesion/Red Marrow
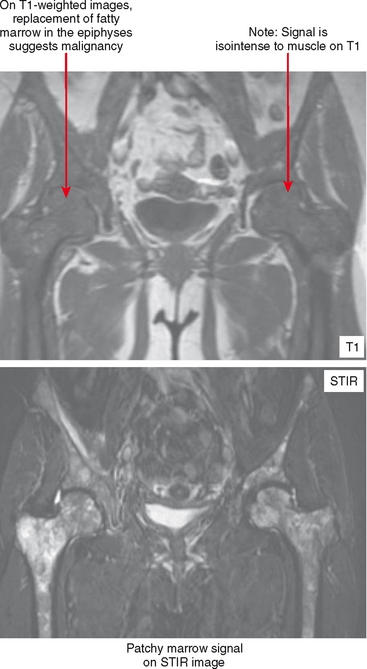
Areas of normally sparce trabeculae can simulate a lytic lesion on radiographs and sometimes on CT (Fig. 6-18). Common locations are the greater tuberosity of the proximal humerus, the proximal femur (called Ward triangle), and the body of the calcaneus. If unclear, MRI can confirm the presence of fat or hematopoietic marrow within these regions.
On MRI, hematopoietic marrow is usually overlooked except when it is perceived on images from a certain sequence (e.g., STIR), when it is in an atypical location (as in the proximal radius), or when it is seen in older persons with heterogeneous replacement with fatty marrow. The key to recognizing hematopoietic marrow is signal and location.
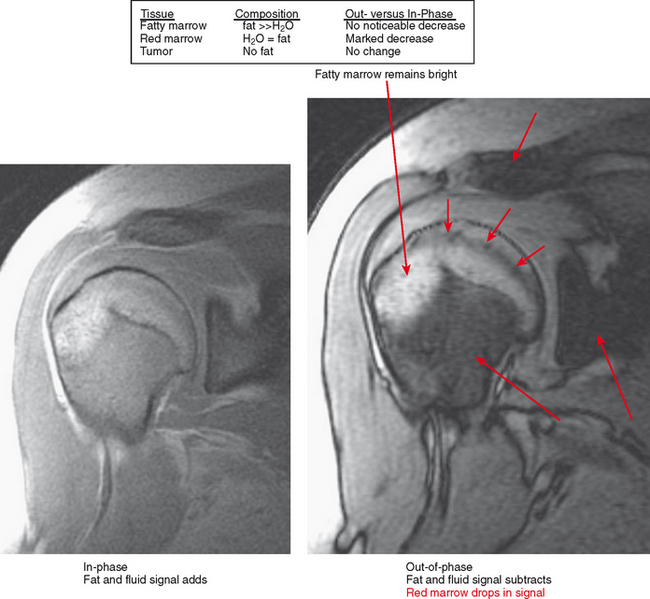
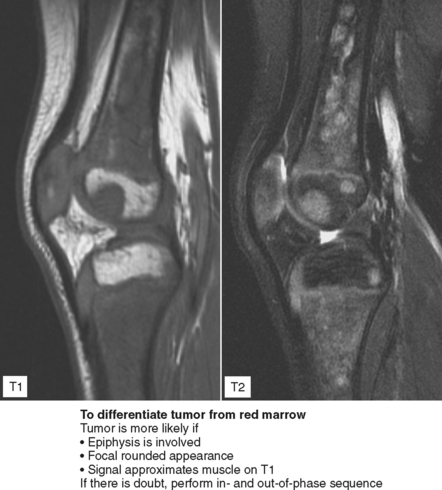
Figure 6-21 Principle for use of in-phase and out-of-phase imaging for differentiation of red marrow from tumor.