Abstract
This chapter discusses different imaging techniques that are increasingly being used in patients with arrhythmias and in cardiac electrophysiology, the latter comprising ablation procedures and implantation of devices. Currently, imaging is mainly used to assess pre-procedural anatomy, allow proper patient selection, procedural guidance, and early recognition of complications. Ongoing developments in both imaging and electrophysiological techniques and their integration will help unravel the complex pathophysiological mechanisms of arrhythmias, and increase the safety and success rate of electrophysiological procedures.
Keywords
Arrhythmia, Imaging, Electrophysiology, Ultrasound, Fluoroscopy, Computed tomography, Cardiac magnetic resonance imaging.
21.1
Introduction
Electrophysiology comprises catheter ablations and cardiac implantable electrical devices (CIED) for malconduction and arrhythmia management, prevention of sudden cardiac death, or cardiac resynchronization, and is a rapidly growing field in cardiology. There is an increased clinical use of advanced cardiac imaging for pre-procedural assessment, procedure guidance, detection of complications, and follow-up in patients with arrhythmias or conduction disturbances ( Table 21.1 ) [ ]. The partly overlapping capabilities, differing diagnostic accuracies, and continuing technological developments require a thorough understanding of the indications, advantages, and disadvantages of different imaging technologies by both cardiac imaging specialists and electrophysiologists. In this chapter, the role of different imaging modalities is discussed focussed on common atrial and ventricular arrhythmias and cardiac resynchronization therapy (CRT).
| Technique | Supraventricular arrhythmias | Ventricular arrhythmias | CIED | Advantage | Limitation |
|---|---|---|---|---|---|
| Routine fluoroscopy | Procedural guidance |
|
| ||
| 3D rotational angiography | Procedural guidance |
|
| ||
| Ultrasound | |||||
| 2D-TTE (3D-TTE and STE may give additional information) |
|
|
| ||
| TOE (3D-TTE may give additional information) |
|
|
| ||
| ICE |
|
|
| ||
| Computed tomography (CT) |
|
|
| ||
| Cardiovascular magnetic resonance (CMR) |
|
|
| ||
| Electroanatomic mapping (EAM) |
|
|
| ||
| 123 I- m IBG—SPECT |
|
|
| ||
| Image integration | |||||
| EAM with CT or CMR |
|
|
| ||
| EAM with ICE |
|
|
| ||
21.2
Fluoroscopy
Catheter ablations are traditionally performed under X-ray fluoroscopic guidance. Rotational angiography is a new technique that enables the creation of real-time 3D images during the ablation procedure. The most important application of both classic X-ray fluoroscopy and rotational angiography is procedural guidance.
21.2.1
Routine fluoroscopy
21.2.1.1
Procedural guidance
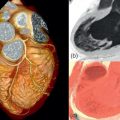
The main role of X-ray fluoroscopy is the guidance of endocardial catheters during ablation procedures of supraventricular and ventricular arrhythmias (see Figure 21.1 ). X-ray fluoroscopy is also used to visualize pacemaker and implantable cardioverter-defibrillator (ICD) leads during implantation. A major concern in relation to radiation exposure is its deterministic and stochastic effects that may lead to observable skin injury [ ] and irreversible radiation damage to DNA. The latter is associated with induction of cancer [ ]. Because of this, several methods have been developed to minimize radiation dose during ablation procedures [ , ]. The use of newer technologies such as non-fluoroscopic three-dimensional navigation systems allow for minimal radiation exposure during catheter ablation [ ].

21.2.2
Rotational angiography
21.2.2.1
Procedural guidance
Three-dimensional rotational angiography (3DRA) was initially introduced as a new modality for 3D imaging of the left atrium (LA) during atrial fibrillation (AF) ablation procedures. To perform this, an X-ray tube mounted on a C-arm performs a 200–240° rotation around the patient while contrast medium is injected in the LA. A large number of 2D projections are acquired during rotation. Reconstruction algorithms transform these 2D images into a set of 3D-reconstructed computed-tomography (CT)-like images. Spatial resolution of 3DRA is comparable or even exceeds that of conventional cardiac CT [ ]. 3DRA enhances lesion identification during AF ablation by improving visualization of the sometimes variable and complex left atrial–pulmonary venous anatomy. 3DRA also allows visualization of surrounding structures such as the esophagus in order to prevent ablation-related fistula formation [ ]. 3DRA has the potential to eliminate the need for pre-procedural CT or cardiac magnetic resonance imaging (CMR). Another important advantage of 3DRA is that it is performed immediately prior to the procedure, thereby avoiding registration errors of the per-procedural catheter position. This may occur when the volume status or position of the patient differs between pre-acquired 3D-roadmap images and the ablation procedure. 3DRA can be performed with acceptable patient radiation dose (5–8 mSv), comparable to cardiac CT [ ]. There is limited data about the use of 3DRA in patients with right ventricular outflow tract ventricular tachycardia (VT) [ ]. ECG-gated rotational angiography is a valuable tool for visualizing coronary venous anatomy during left ventricular lead implantation procedures [ ].
21.3
Ultrasound
21.3.1
Transthoracic echocardiography
Two-dimensional echocardiography (2DE) is widely available, safe, and cost-effective. It remains an important initial investigation, because arrhythmias are often associated with underlying heart disease and reduced left ventricular ejection fraction (LVEF). It is also useful to detect certain complications of electrophysiological procedures and as a tool for follow-up. Speckle tracking echocardiography (STE) is a novel technique that more accurately determines myocardial deformation (strain) than tissue Doppler methods. Three-dimensional echocardiography (3DE) overcomes the limitations of 2DE derived geometric assumptions, and has become an established method to determine chamber volumes and function [ ].
21.3.1.1
Pre-procedural assessment
AF, atrial flutter, or atrial tachycardia, is often associated with hypertension (70%), valvular heart disease (36%), coronary artery disease (28%), and cardiomyopathy (10%) [ ]. Catheter ablation with electro-isolation of pulmonary venous (PV) foci that frequently trigger AF is an effective treatment option for patients with symptomatic and drug-refractory AF [ ]. Although less well suited than CT or CMR, 2DE can give pre-procedural information on atrial and PV anatomy and left atrial (LA) appendage thrombus.
2DE derived parameters predict the success of therapy, i.e., LA-size ≥ 50 mm (from M-mode) increases the risk of AF recurrence both after cardioversion and ablation [ ] [ ]. 3DE more accurately assesses LA volume than 2DE approaches, but cut-off values that predict procedural success after ablation are currently unknown [ ]. Decreased STE-derived LA global and regional strain may become important pre-procedural determinants to predict AF recurrence after ablation. 2DE can also be used to risk stratify patients with AF, because LVEF ≤ 40% is strongly associated with stroke [ , ].
Sudden cardiac death (SCD) remains a leading cause of death worldwide. All patients with serious ventricular arrhythmias should undergo 2DE because of the association with underlying cardiomyopathy. 2DE should also be performed in all patients scheduled for VT ablation to rule out intracardiac thrombus.
LVEF determination after myocardial infarction (MI) is important and incorporated in current international guidelines because echocardiography derived cut-off values are used for prophylactic CIED implantation (either CRT, and/or ICD) [ ]. Despite this, considerable risk heterogeneity exists among patients with low LVEF, and a reduced LVEF is a non-specific predictor of future life-threatening arrhythmias. Less than 50% of patients with prior MI who die suddenly have LVEF < 30%. This indicates that improved risk stratification to determine which patient is at greatest risk for SCD and which patient will likely not benefit form ICD therapy is urgently needed. Malignant arrhythmias are the result of a complex interaction between heterogeneity in scar tissue and regional electrophysiological properties. Mechanical dispersion of 70 ms using STE is a strong and independent predictor for identifying arrhythmic events (65% sensitivity and 92% specificity) [ ]. The risk for arrhythmic events increases with more extensive wall motion abnormalities (HR 2.18, 95% CI 1.03–4.65, P = 0.04, for every 1-point increase in wall motion score index), restrictive filling pattern, and inducible myocardial ischemia [ ].
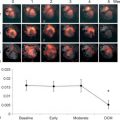
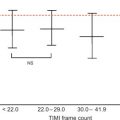
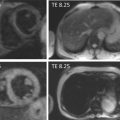
CRT is an effective therapy for patients with drug-refractory end-stage heart failure, low LVEF (≤ 35%), and wide QRS complex (≥ 120 ms). Eligible patients should undergo careful pre-implantation imaging to evaluate cardiac anatomy, LV size, and function to predict long-term clinical outcome [ ]. 2DE has an advantage over other diagnostic modalities that determine EF, because it is widely available and provides valuable information on concomitant valvular disease, diastolic function, and volume status. Preferentially, volumetric methods (Simpson’s biplane or 3DE) instead of biplane methods should be used to assess LV volumes and EF [ ]. Although 2DE quantification of mechanical dyssynchrony using tissue Doppler imaging emerged as a promising technique to predict the response of CRT, it failed to show a consistent benefit in larger prospective multicenter trials [ ]. The systolic dyssynchrony index (SDI), a 3DE-derived parameter of dyssynchrony (10.7 ± 3.6% in patients vs. 2.7 ± 0.9% in healthy volunteers; P < 0.001; see Figure 21.2 ), or STE-derived delays (≥ 130 ms between anteroseptal and posterior wall using radial and transverse strains; see Figure 21.3 ) are novel methods to predict response to CRT but need further prospective multicenter investigation [ , ].


21.3.1.2
Procedural guidance
2DE has no role in the procedural guidance during ablation of (supra) ventricular arrhythmias or CIED implantation. However, tissue Doppler imaging of the atria using 2DE holds promise as a non-invasive tool to determine AF cycle length, allowing the effect of antiarrhythmic therapy to be monitored [ ].
21.3.1.3
Complications
Pericardial effusion (PE) and cardiac tamponade may occur in up to 14% after CIED implantation and up to 6% of arrhythmia ablations, depending on technique used, and concomitant antiplatelet or anticoagulant therapy [ , ]. PE after ablation for AF may be related to the transseptal puncture, ablation-related damage to the LA or myocardial or coronary venous perforation during CIED implantation. 2DE is well suited to detect PE and cardiac tamponade and should be performed as soon as there is a suspicion (chest discomfort), even in the absence of hemodynamic abnormalities. Although these complications typically develop during the procedure, they may occur days later as well, particularly if anticoagulant therapy is restarted.
PV stenosis after AF catheter ablation is a rare complication, which is mostly mild and clinically silent. Although CT and CMR are the diagnostic modalities of choice, 2DE can detect increased flow velocities in the PV with color and pulsed wave Doppler. Tricuspid valve insufficiency after electrophysiological procedures and CIED implantation can be recognized early with transthoracic echocardiography (TTE). It carries an adverse prognosis and may be corrected by repositioning the lead. CIED-related endocarditis occurs in 0.3–0.4/1000 [ ]. Although TEE is superior, 2DE can be helpful to diagnose right- (lead-related) and left-sided endocarditis. Importantly, a negative 2DE in the setting of high clinical suspicion and positive blood cultures necessitates further investigation.
21.3.1.4
Follow-up
Studies suggest that after successful ablation, LA enlargement reverses and contraction and reservoir function improve (reverse structural remodeling) in so-called responders (i.e. ≥ 15% decrease in maximum LA volume). Because responders suffer fewer AF recurrences, LA reverse remodeling may become a surrogate marker of successful AF ablation. In a study of 144 patients undergoing catheter ablation for AF, responders (63%) had a significant decrease in maximum LA volumes (from 31 ± 7 to 22 ± 6 ml/m 2 ), whereas non-responders showed an increase (29 ± 5 to 31 ± 6 ml/m 2 ) after 13.2 ± 6.7 months. Two-thirds of responders remained in sinus rhythm, whereas one-third had recurrent AF [ ].
TTE plays a role in the serial follow-up of patients after CRT to assess ventricular function and remodeling, which is characterized by a reduction in LV volumes and improvement in LVEF after 3–6 months. However, only two-thirds of patients respond to CRT and improve in terms of heart failure symptoms, functional status, and cardiac function. Although individual optimization of atrioventricular (AV) and ventriculoventricular (VV) delays after CRT using Doppler techniques (mitral inflow, aortic velocity time integral) have shown to improve hemodynamic function acutely [ ], the role of routine optimization in all patients is controversial. There are little to no data that support long-term clinical improvement after routine CRT optimization. In the SMART-AV trial, comparing a fixed nominal AV delay of 120 ms, CRT device-determined (SmartDelay), and echocardiography-determined AV optimization, no difference in improvement in LV end-systolic volume, LVEF, and functional measures was found after 6 months [ ]. Despite the availability of numerous AV optimization methods, only the simple and iterative method of finding the optimal AV delay that optimizes diastolic filling is recommended in non-responders or in advanced diastolic dysfunction [ , ]. The role of VV optimization is even less clear.
21.3.2
Transesophageal echocardiography
Transesophageal echocardiography (TOE) is a widely available, safe, and reliable technique to evaluate cardiac chambers, major vessels, and cardiac and valvular function. When carried out by experienced operators, it is associated with a very low risk (below 0.05%) of complications [ , ]. TOE is mainly carried out after routine TTE and offers more detailed information of structures close to the esophagus than TTE. In recent years, three-dimensional TOE (3D-TOE) has become available by most vendors [ ] and provides 3D overviews of cardiac structures. 3D-TOE is useful to visualize complex structures such as the inter-atrial septum during punctures, the left atrial appendage (LAA), and tracking of catheters.
21.3.2.1
Pre-procedural assessment
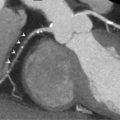
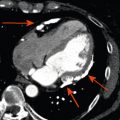
AF is associated with an increased risk of atrial thrombus formation. Presence of LA thrombus (see Figure 21.4 ) is considered a contraindication for performing (electrical) cardioversion or intracardiac ablation of atrial tachyarrhythmias. TTE offers information on risk factors associated with thrombus formation, such as LA size or depressed LV function, but lacks specificity and sensitivity for thrombus detection in comparison to TOE [ ]. TOE is considered the gold standard to exclude LA thrombus prior to catheter ablation [ ]. Spontaneous echo contrast on TOE is also considered a risk factor for embolic stroke in patients with AF. TOE prior to cardioversion may decrease the risk of hemorrhagic stroke when compared to a strategy using oral anticoagulation alone [ ]. In the workup of patients with ventricular arrhythmias, SCD prevention, or CRT, TOE can be useful in patients with LV dysfunction related to underlying valvular disease, with respect to mechanism and additional (surgical) treatment options [ ].

21.3.2.2
Procedural guidance
Intraprocedural cardioversion can be safely performed while the TOE probe remains in place. TOE helps guide the puncture of the inter-atrial septum and tracking of catheters and safe guide wire placement in the pulmonary veins (PV), thereby avoiding unnecessary complications [ ]. Peri-procedural guidance with TOE is advised in challenging cases, such as redo-procedures with increased septal thickness [ ], after atrial septal closure device placement, or in grown-up congenital heart disease [ ]. LAA closure devices (for instance WATCHMAN™) are being used to prevent thrombotic complications in patients with AF who have contraindication for anticoagulation therapy [ ]. Placement of such devices is performed under TOE guidance to ensure correct sizing and placement and to identify early complications during or after device placement. Finally, recent literature suggests that 3D-TOE guided PV isolation without fluoroscopy is feasible [ ].
Lead placement for CRT is routinely carried out under fluoroscopic guidance, although (3D) TOE may be useful in the localization of the coronary sinus. TOE guided transseptal puncture can help in endocardial left ventricular lead placement in patients with unfavorable venous anatomy or failed epicardial lead placement [ , ].
21.3.2.3
Complications
After transseptal puncture, TOE allows early intraprocedural detection of complications such as PE. When clinical suspicion of PE remains high, TOE should follow a negative TTE examination. With current ablation techniques such as cryoablation, PV stenosis is becoming increasingly rare. As with TTE, TOE may detect increased Doppler flow velocities, but CT and CMR remain the most appropriate techniques to detect this complication. CIED-associated infections have a poor prognosis, and prompt lead removal is generally advised [ ]. TOE has superior sensitivity to TTE for detection of endocardial lead infection. Although a negative initial TOE does not rule out CIED infection, complications can readily be visualized [ ]. A recent study showed that a lead-associated mass occurred in 14% of patients after CIED placement using TOE. However, 72% of these patients did not have any evidence of infective endocarditis. Thus, consideration should be given before routinely removing leads that have masses attached [ ].
21.3.2.4
Follow-up
TOE may be considered for detecting late complications after electrophysiological procedures when TTE has substandard image quality, but does not play a role in routine follow-up.
21.3.3
Intracardiac echocardiography
21.3.3.1
Procedural guidance
In contrast to TEE, intracardiac echocardiography (ICE) represents a purely peri-procedural tool for guidance and safety of catheter-based procedures. ICE permits direct imaging of all structures relevant to a transseptal puncture. The individual components of the atrial septum—septum primum and septum secundum, the relationship of the fossa ovalis to the aortic valve and ascending aorta, and the posterior atrial wall—can all be readily visualized. ICE guided transseptal puncture may abandon the need for fluoroscopic guidance of catheters, thereby decreasing radiation burden associated with electrophysiological procedures [ ]. In addition, ICE causes less patient discomfort as compared to TOE, decreasing the need for peri-procedural sedation. Newer generation phased array-ICE probes produce wedge-shaped images and even allow (color) Doppler imaging that can be post-processed on conventional echocardiographic workstations. PV dimensions and LAA thrombus can be detected reliably, especially when employing multiple ICE views [ ]. These advantages and a low complication rate (3.3% in a single-center registry of 1192 ICE guided procedures) [ ] have led to the widespread use of ICE during electrophysiological procedures.
Three-dimensional reconstruction of ICE images is feasible, and may be used for electroanatomical mapping studies (see also Section 21.6 ). Future developments in real-time 3D ICE may further assist complicated transseptal puncture and catheter guidance.
21.3.3.2
Complications
An important advantage of ICE during electrophysiological procedures is the continuous surveillance of complications. Early recognition of PE may lead to anticoagulation reversal and prevent cardiac tamponade. ICE also allows guiding subsequent pericardiocentesis. Future studies comparing accuracy, reproducibility, and clinical outcome of ICE guidance versus TOE versus isolated fluoroscopy is needed to adopt ICE imaging as a primary nonradiographic imaging tool during electrophysiological procedures [ ].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree