Acute ischemic stroke affects 3.3 of 100,000 children per year. The causes of AIS in children can be broadly divided into the following 6 categories: cardiac disese, sickle cell disease, moyamoya, arterial dissection, other arteriopathies, and other causes. Approximately 24% of the cases are classified as idiopathic. Magnetic resonance imaging (MRI) and cerebral angiography play an important role in the determining the causes of an AIS in children. Medical approaches, including anticoagulation, anti-inflammatories, and antiplatelet therapies, surgical revascularization and endovascular approaches may have a role in the management of AIS in children.
Key points
- •
Nonatheromatous abnormalities of the cerebral and/or cervical arterial circulation are very common in childhood arterial ischemic stroke and are the most important determinants of treatment and prognosis.
- •
Intracranial disease is far commoner than cervical disease but imaging needs to include the aortic arch to the circle of Willis.
- •
There are standardized disease definitions that should be used precisely to promote accuracy of diagnosis.
- •
Key diagnostic dilemmas are the identification of vasculitis and moyamoya.
- •
Hyperacute thrombolysis is unlikely to be an appropriate treatment for most children with arterial ischemic stroke but may have a role in selected patients.
- •
As well as medical approaches, including anticoagulation, anti-inflammatories, and antiplatelet therapies, surgical revascularization and endovascular approaches may have a role in the management of these diseases.
Introduction
Arterial ischemic stroke (AIS) affects 3.3 of 100,000 children per year, with residual morbidity in two-thirds of patients, resulting in significant health care, personal, and social costs. The cause of AIS is commonly multifactorial, with one-half of the affected children having a premorbid illness, such as congenital or acquired heart disease, vascular disorders, or an infection. With the advent of noninvasive cerebrovascular imaging, especially magnetic resonance angiography (MRA), it has become apparent that most affected children have abnormalities, or arteriopathies, of the cerebral or cervical arterial circulation. This article discusses the clinical presentation, epidemiology, and cause of AIS in children. Then, diagnostic imaging, treatment options, and outcomes as they relate to AIS in children are discussed. Hemorrhagic stroke in children is not included in this article.
Introduction
Arterial ischemic stroke (AIS) affects 3.3 of 100,000 children per year, with residual morbidity in two-thirds of patients, resulting in significant health care, personal, and social costs. The cause of AIS is commonly multifactorial, with one-half of the affected children having a premorbid illness, such as congenital or acquired heart disease, vascular disorders, or an infection. With the advent of noninvasive cerebrovascular imaging, especially magnetic resonance angiography (MRA), it has become apparent that most affected children have abnormalities, or arteriopathies, of the cerebral or cervical arterial circulation. This article discusses the clinical presentation, epidemiology, and cause of AIS in children. Then, diagnostic imaging, treatment options, and outcomes as they relate to AIS in children are discussed. Hemorrhagic stroke in children is not included in this article.
Clinical presentation
The clinical presentation of an AIS in children varies according to the age of the child, the cause, and the involved artery. In general, embolic episodes tend to present suddenly, whereas a stenosis or thrombosis may have a more gradual onset. Focal neurologic deficits such as hemiplegia are the most common presentation of AIS in children. Other signs and symptoms, such as headache, language and speech difficulties, mood or behavior disturbances, and seizures, are less specific. In children less than 1 year of age, seizures and encephalopathy are more common than focal neurologic signs. Stroke in the posterior circulation can present as vertigo, ataxia, and vomiting. In infancy, a typical presentation includes seizure, lethargy, and/or apnea often without a focal neurologic deficit. Braun and colleagues found that children with nonarteriopathic AIS were significantly more likely than those with arteriopathy to have an abrupt onset of symptoms.
Incidence
The reported incidence of childhood stroke has increased in the last 20 years according to population studies, most likely related to improvements in neuroimaging techniques. Schoenberg and colleagues studied stroke in children in Rochester, MN, from 1965 through 1974. They found an estimated average annual incidence rate of 1.89 of 100,000 and 0.63 of 100,000 per year for hemorrhagic and ischemic strokes, respectively. Their overall average annual incidence rate was 2.52 of 100,000 per year. Broderick and colleagues in the 1990s, studying childhood stroke in metropolitan Cincinnati, found an overall incidence of 2.7 of 100,000 per year. Surprisingly, hemorrhagic stroke (incidence 1.5/100,000/y) was shown to be more frequent than ischemic stroke (incidence 1.2/100,000/y). Updated incidence figures from the Canadian Pediatric Ischemic Stroke Registry showed that ischemic stroke in childhood occurs in 3.3 of 100,000 per year, with 2.6 of 100,000 per year and 0.7 of 100,000 per year as the rates for arterial and sinovenous strokes, respectively. When the authors included hemorrhagic strokes, the overall childhood stroke incidence exceeded 6 of 100,000 per year. A retrospective analysis of a California-wide hospital discharge database estimated an incidence of stroke (AIS, cerebral venous thrombosis, and hemorrhagic stroke including subarachnoid hemorrhage) of 2.3 of 100,000 per year and a prospective study across the United Kingdom and Ireland reported an incidence of stroke (AIS, cerebral venous thrombosis, and hemorrhagic stroke including subarachnoid hemorrhage) of 2.3 of 100,000 per year. Adverse outcomes include death in 10%, neurologic deficits or seizures in 70%, and recurrent strokes in more than 20% of children affected with a stroke. Strokes in children can result from either vascular occlusion (ischemic stroke) or from bleeding caused by a ruptured blood vessel (hemorrhagic stroke). The incidence of ischemic versus hemorrhage stroke is different in children versus adults. Ischemic stroke accounts for 55% of strokes in children, whereas it causes 85% of adult strokes. Boys have a higher risk of pediatric stroke than girls, with the type of stroke varying with age. Infants (30 days to 1 year) have a higher reported rate of ischemic stroke, whereas older children (15–19 years) show a higher incidence of hemorrhagic stroke. Black children have more than twice the risk of stroke of white children. Hispanic children have a lower risk that white children, and Asian children show a roughly equal risk.
Etiology
There are major differences in the risk factors for stroke in children compared with adults. Compared with adults, children are more likely to have an infectious or inflammatory disorder underlying the stroke. The causes of AIS in childhood are often multifactorial and different than in adults. The most fundamental difference between strokes in children and adults is the wide array of risk factors seen in children versus adults ( Box 1 ). The causes of AIS can be broadly divided into the following 6 categories: cardiac disease, sickle cell disease, moyamoya, arterial dissection, other arteriopathy, and other causes. Approximately 24% of the cases are classified as idiopathic.
Heart disease
Congenital heart disease
Rheumatic heart disease
Cardiomyopathy
Endocarditis/myocarditis
Dysrhythmias
Hematologic disorders
Hemoglobinopathy (sickle cell disease)
Polycythemia
Thrombocytosis
Leukemia/lymphoma
Coagulopathies
Protein C/S deficiency
Antithrombin III deficiency
Antiphospholipid antibody syndrome
Lupus anticoagulant
Factor V Leiden mutation
Disseminated intravascular coagulation
Metabolic
Mitochondrial disorders
Homocystinuria/hyperhomocysteinemia/Fabry disease
Dyslipidemias
Vasculopathy
Moyamoya syndrome
Fibromuscular dysplasia
Neurocutaneous disorders
Vasculitis
Connective tissue diseases
Henoch-Schonlein purpura
Polyarteritis nodosa
Kawasaki disease
Trauma to the head or neck
Infection
Meningitis
Varicella
HIV
Idiopathic
Cardiac Abnormalities
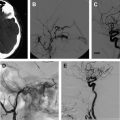
Cardiac procedures, including catheterization, extracorporeal membrane oxygenation, and surgery, are all considered risk factors for childhood AIS. These cardiac procedures are all likely mediated from a heart embolus. Congenital heart disease ( Fig. 1 ), valvular heart disease, cardiac arrhythmias, and cardiomyopathy are also risk factors for childhood AIS. Abnormalities of the cardiac valves, major vessels, and myocardium may result in turbulent blood flow and the formation of a thrombus that can embolus to the cerebral vessels, which is particularly a problem in children with a right-to-left shunt. In a retrospective national database review of children with AIS, the most frequent comorbidity was noted to be congenital heart disease. Almost 25% of children diagnosed with AIS in the Canadian Registry had cardiac disease at presentation. In another childhood AIS cohort study, 28% of the children had cardiac abnormalities.
Hypertension may also be a risk factor for childhood AIS. Ganesan and colleagues showed an association with vasculopathy and systolic hypertension. Lo and colleagues also found that hypertension was a risk factor for pediatric stroke. It is hypothesized that hypertension in children may be related to chronic anemia, comorbid medical conditions, or a systemic response to an acquired vasculopathy.
It is uncertain if a patent foramen ovale (PFO) is a significant risk factor for childhood AIS. In 4 of 6 children with idiopathic AIS, transcranial Doppler with Valsalva suggested a right-to-left shunt. PFO is a known risk factor for AIS in the adult literature; however, the incidence of a PFO in healthy children is unknown.
Sickle Cell Disease
Sickle cell disease (SCD) is one of the most common causes for AIS in childhood, particularly among individuals such as African Americans, of recent historical descent from populations inhabiting malaria-endemic regions. The prevalence of cerebrovascular events in patients with SCD is reported to be 4.0% and the incidence 0.6 per 100 patient-years. The highest risk for stroke is between the ages of 2 and 5 years of age. The risk of recurrence is as high as 67%. AIS in SCD is largely related to occlusive disease of in the terminal internal carotid artery (TICA)/proximal middle cerebral artery (MCA)/anterior cerebral artery (ACA), which is caused by endothelial injury from the deformed erythrocytes. Other factors, such as anemia and hypoxemia, may additionally predispose to AIS. Pathologic features of SCD are often associated with thrombotic occlusion and the presence of collateral vessels. Blood transfusion, aiming to reduce the circulating level of Hemoglobin S (HbS) and correct anemia sufficiently to suppress bone marrow production of HbS, is extremely effective in both primary and secondary prevention of AIS. The standard of care in many countries is now to institute screening for cerebrovascular disease in children with SCD using transcranial Doppler ultrasound from the age of 2 years, at annual intervals, and instituting blood transfusion for those with abnormally elevated velocity in the middle cerebral arteries. Blood transfusion is also recommended for children who have had clinical AIS, for secondary prevention. Unfortunately, despite its efficacy, blood transfusion places a major burden on the child and family, as well as carrying medical risks such as blood-borne infection. The logistics of blood transfusion also make it impossible in resource-poor settings. There is ongoing research into the role of other therapies, such as hydroxyurea. Bone marrow transplantation may be considered in children with AIS but the major hurdle with this is identifying a suitable donor. Patients with SCD who have a moyamoya morphology of occlusive disease terminal internal carotid artery (TICA), M1 segment of the middle cerebral artery (M1), A1 segment of the anterior cerebral artery (A1) (TICA/M1/A1 occlusive disease with basal collaterals) are at highest risk of recurrence. Surgical revascularization may have a role in such individuals but the role of surgery in the context of the overall management of SCD is as yet unclear.
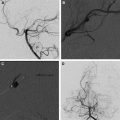
Moyamoya
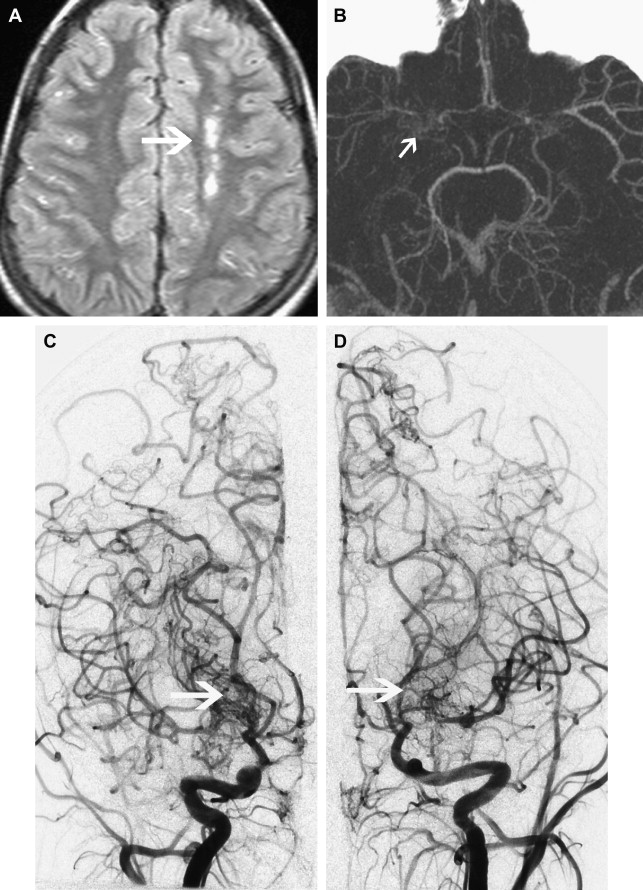
Moyamoya is a radiological term characterized by bilateral stenosis of the distal internal carotid arteries with lenticulostriate collateralization that results in the pathognomonic “puff of smoke” appearance ( Fig. 2 ) on conventional cerebral angiogram and is diagnosed in up to 20% of childhood AIS. The condition is common in Japan and East Asia, where familial cases are also clearly recognized. Currently several genetic loci have been implicated in these. The disease definition relates to Japanese patients and the degree of radiological and phenotypic overlap between them and patients of other ethnicities is not clear. Moyamoya may be apparently idiopathic (moyamoya disease) or secondary to a large variety of other conditions (moyamoya syndrome), including trisomy 21, neurofibromatosis type 1, and cranial irradiation. This wide spectrum of associations suggests multiple mechanisms are implicated in the genesis of the arteriopathy. Unfortunately the term is used very loosely in clinical practice and in the literature and is commonly applied in cases of bilateral TICA disease, whether or not the other radiological features are present. The genetics literature describing moyamoya as a feature of many disorders is likely, in fact, to reflect a variety of entities. An example of this is the very distinctive radiological phenotype of cerebral arteriopathy associated with mutations in the alpha-actin-2 (ACTA2) gene, initially described as “moyamoya.” In contrast, these patients have several distinctive radiological features, namely proximal ectasia, distal occlusive disease, absence of basal collaterals, and an unusually straight morphology of the intracranial arteries. Because this mutation is dominantly inherited and predisposes to aortic aneurysms, it is an important condition to identify by the radiological signature. There are likely to be further examples of distinctive phenotypes, currently subsumed under imprecise application of the term “moyamoya.”
Arterial Dissection
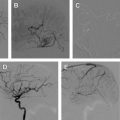
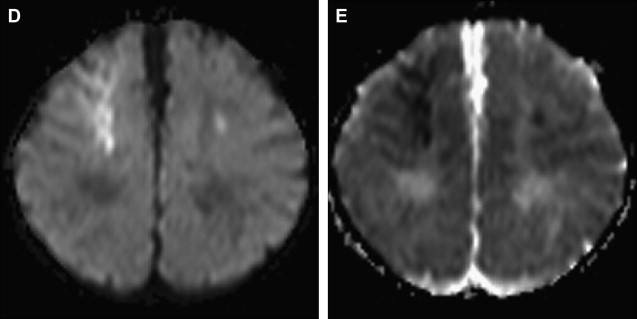
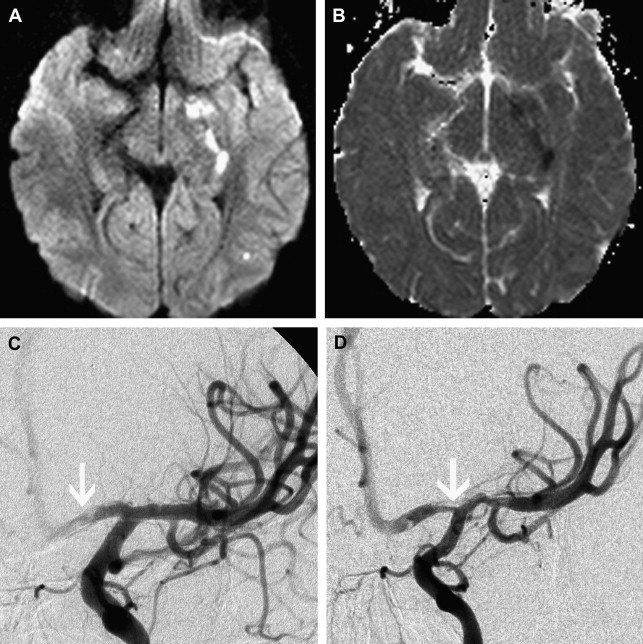
Dissection of the cervical arterial arteries is said to account for 15% to 20% of AIS in children; this is largely seen in older children and adolescents and is usually spontaneous. Cases may be attributed to significant or even mild traumatic events, and some to connective tissue disorders, such as Ehlers-Danlos or Marfan syndrome. The radiological features required to diagnose dissection can be elusive; absolute evidence comes from visualization of a dissection flap, double vascular lumen, or hematoma within the arterial wall. Indirect evidence includes tapering vascular occlusion (string sign) or irregular caliber change. Vertebral artery (VA) dissection is the most common risk factor for posterior circulation AIS in children and should be actively excluded, by catheter angiography if necessary, unless there is a clear alternative diagnosis ( Fig. 3 ). The V3 loop of the VA is the commonest site of dissection and is often not clearly visualized on MRA. Abnormalities of the cervical spine (eg, calcified arcuate ligament) may be associated with VA dissection and should be excluded by plain radiographs of the neck in flexion and extension. Dissection of the intracranial arteries undoubtedly exists but is difficult to identify and distinguish from other entities, such as focal cerebral arteriopathy (FCA), with confidence. The importance of identifying arterial dissection is that it is one of the diagnoses which, based on current treatment recommendations, alters the treatment approach. Furthermore, the literature suggests that patients with an index event are at increased risk of recurrent dissection in the future. It is likely that affected patients have an underlying genetically determined predisposition, potentially exacerbated by environmental triggers, such as infection or trauma.
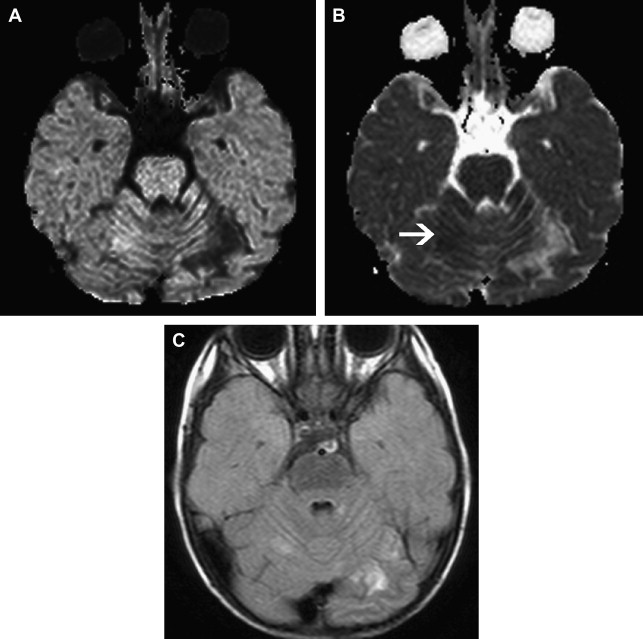
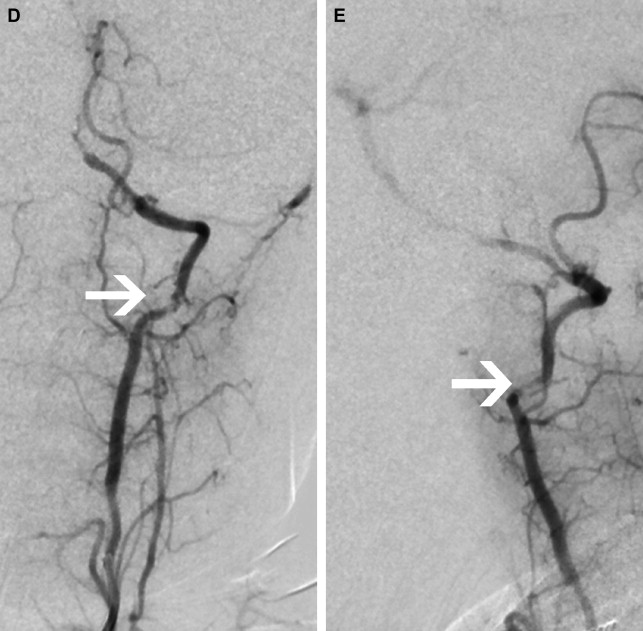
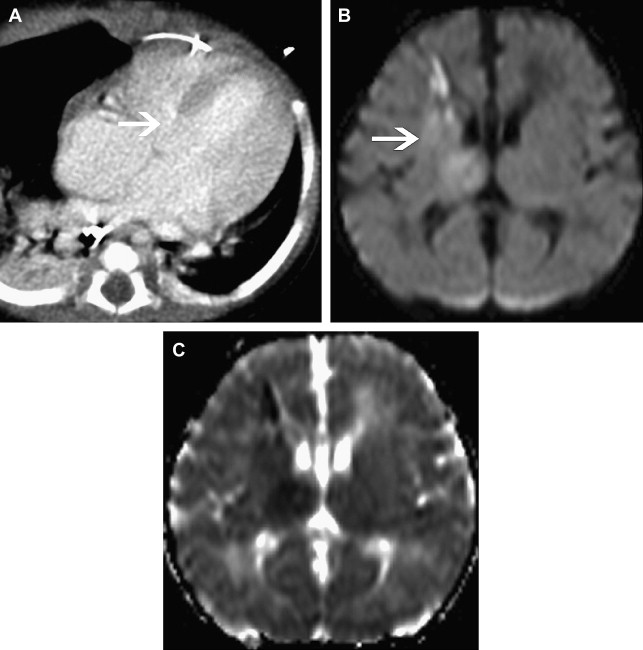
Focal Cerebral Arteriopathy of Childhood
To promote consistency, the International Pediatric Stroke Study group has advocated application of standard disease definitions. The most commonly identified cerebral arteriopathy in childhood is focal occlusive disease of the TICA/proximal MCA or ACA; this morphology of disease was originally termed “transient cerebral arteriopathy,” a definition that required demonstration of disease stability or regression over time. More recently, this term has been superseded by the term “focal cerebral arteriopathy of childhood” (FCA) ( Fig. 4 ) to refer to this pattern of focal proximal occlusive disease identified at a single time point. The FCA pattern of arteriopathy is commonly seen in children after varicella cerebral infarction and in these cases it is hypothesized that the disease represents a focal inflammatory response to the viral infection. The role of infection and vascular inflammation in the genesis of arteriopathy in other cases remains controversial; however, there does seem to be an increased rate of antecedent upper respiratory tract infection in children with FCA. The distinction between FCA and primary angiitis of the central nervous system (PACNS) is controversial. Although vascular inflammation is thought to be a disease mechanism common to both entities, they are distinguished by their clinical course, with PACNS typically having a multifocal and progressive course, in contrast to the monophasic course observed in FCA. Currently there are no biomarkers that robustly distinguish between FCA and PACNS and there is confusion in the literature, with cases that appear identical in terms of imaging being cited as examples of one or another entity. Catheter angiography may be useful in cases of suspected PACNS if there is multifocal disease affecting smaller arteries. If this is undertaken, it is worth considering systemic angiography, looking for evidence of systemic vascular involvement.
Inflammatory Arteriopathy
Inflammatory arteriopathy of the central nervous system may be part of a systemic vasculitis or isolated PACNS. A variety of vessel sizes may be involved; small vessel vasculitis (confirmed histologically) may be associated with normal catheter angiography. Central nervous system vasculitis is notoriously difficult to identify and no investigation, including leptomeningeal biopsy, has a sensitivity of more than 40%. Thus the diagnostic approach has to be broad, including serology and cerebrospinal fluid (CSF) examination, looking for evidence of disease in other organ systems. It is important to have a high level of clinical vigilance, especially in patients with recurrent AIS, multifocal infarction, progressive arteriopathy, or a more diffuse, encephalopathic presentation.
Idiopathic—Other Causes
Although the Sebire classification captures most cerebral arteriopathies seen in association with AIS, it is not entirely comprehensive. In the context of a specialist pediatric neurovascular service, a significant proportion of patients cannot be placed in a category using this scheme; common features in them are multifocal arteriopathy ( Fig. 5 ), involvement of other organs (especially renal arteries), a genetic or syndrome diagnosis, and the coexistence of occlusive and aneurysmal cerebrovascular disease in the same individual. It is likely that these patients have genetically mediated disease, potentially exacerbated by environmental triggers.
Genetic/Metabolic Conditions
Inherited metabolic disorders are associated with childhood AIS. Some examples include Fabry disease, an X-linked deficiency of α-galactosidase; cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy; Menkes syndrome, a copper transport disorder; and homocystinuria, a deficiency of cystathionine-β-synthase that results in hyperhomocysteinemia.
Sympathomimetic Drugs
Recreational drugs in adolescents is a possible cause of childhood AIS. AIS may be associated with cocaine or amphetamine use related to hypertension and vasospasm. It is unclear whether other sympathomimetic agents used in the treatment of migraine headaches or attention deficit disorder are a risk factor for AIS.
Hypercoagulable States
Several prothrombotic conditions seem to contribute to the risk of childhood AIS. A meta-analysis of 22 observational cohort studies showed a significant association between the first AIS and the following prothrombotic conditions: inherited deficiency of protein C, protein S, antithrombin III, factor V Leiden, factor II methylene tetrahydrofolate reductase polymorphism, elevated level of lipoprotein (a), and the presence of antiphospholipid antibodies. Thrombophilia may also be an independent risk factor for AIS.
In a study of 50 children with AIS followed for a mean duration of 21 months, elevated d -dimer levels were noted most commonly in children with cardiac disease. d -dimer is a marker of coagulation activation, suggesting an underlying thrombophilia state in cardiac disease, which was also identified in children with AIS and cardiac disease by Strater and colleagues.
Additional hematologic factors that may confer an increased risk of AIS include SCD, iron deficiency anemia, thrombocytosis, and polycythemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree