Arterial dissections of head and neck arteries were first identified pathologically in the 1950s, but not until the 1970s and the 1980s did they begin to be widely recognized as a clinical entity. Carotid and vertebral artery dissections account for only 2% of all ischemic strokes, but they account for approximately 20% of thromboembolic strokes in patients younger than 45 years. The cause of supra-aortic dissections can be either spontaneous or traumatic. This article addresses spontaneous cervical and cerebral artery dissections.
Key points
- •
Cervicocranial dissections are thought to be spontaneous if no evidence of preceding trauma is found.
- •
They occur primarily in middle-aged men with a yearly incidence of 2.6 per 100,000 for carotid artery dissection and 1 to 1.5 per 100,000 for vertebral artery dissections.
- •
Ischemic symptoms are often delayed in onset compared with the dissection event.
- •
Arterial dissection often heal with medical management within 3 to 6 months, and associated aneurysms tend to stabilize or even decrease in size and completely resolve in some cases.
- •
The risk of recurrent spontaneous dissection is 2% within the first month and decreases to 1% 1 year thereafter.
- •
No level I evidence is available to guide the treatment of this disease.
- •
Antithrombotic therapy is typically started in the acute phase of a cervicocerebral arterial dissection unless specific contraindications are present.
- •
For extracranial arterial dissections, endovascular management is usually reserved for cases in which medical therapy fails.
- •
For intracranial arterial dissections, cases with hemorrhagic presentation are best treated with trapping of the dissected segment either endovascularly or surgically with or without a bypass. This approach offers the lowest risk for recurrent hemorrhage. For nonhemorrhagic presentation, surgical and/or endovascular approaches are typically reserved for failed medical therapy.
Introduction
Arterial dissections of head and neck arteries were first identified pathologically in the 1950s, but not until the 1970s and the 1980s did they begin to be widely recognized as a clinical entity. Carotid and vertebral artery dissections account for only 2% of all ischemic strokes, but they account for approximately 20% of thromboembolic strokes in patients younger than 45 years. The cause of supra-aortic dissections can be either spontaneous or traumatic. A dissection is deemed spontaneous if no evidence of preceding trauma is found. In some cases, the preceding traumatic event might be missed because of its benign nature (eg, violent coughing or simple neck manipulations). Therefore, these cases might be misdiagnosed as spontaneous dissections. This article addresses spontaneous cervical and cerebral artery dissections.
Because of the inconsistent use of terminology throughout the literature and to avoid any confusion, this article uses the following definitions:
- •
Dissection: a tear alongside the internal wall of an artery with extravasation of blood between the intima and media, with potential luminal narrowing.
- •
Dissecting aneurysm: aneurysmal dilatation caused by dissection of blood between the media and the adventitia.
- •
Pseudoaneurysm: leaking of blood outside of the artery with subsequent confinement of the extravascular hematoma; may or may not produce narrowing of the lumen.
Introduction
Arterial dissections of head and neck arteries were first identified pathologically in the 1950s, but not until the 1970s and the 1980s did they begin to be widely recognized as a clinical entity. Carotid and vertebral artery dissections account for only 2% of all ischemic strokes, but they account for approximately 20% of thromboembolic strokes in patients younger than 45 years. The cause of supra-aortic dissections can be either spontaneous or traumatic. A dissection is deemed spontaneous if no evidence of preceding trauma is found. In some cases, the preceding traumatic event might be missed because of its benign nature (eg, violent coughing or simple neck manipulations). Therefore, these cases might be misdiagnosed as spontaneous dissections. This article addresses spontaneous cervical and cerebral artery dissections.
Because of the inconsistent use of terminology throughout the literature and to avoid any confusion, this article uses the following definitions:
- •
Dissection: a tear alongside the internal wall of an artery with extravasation of blood between the intima and media, with potential luminal narrowing.
- •
Dissecting aneurysm: aneurysmal dilatation caused by dissection of blood between the media and the adventitia.
- •
Pseudoaneurysm: leaking of blood outside of the artery with subsequent confinement of the extravascular hematoma; may or may not produce narrowing of the lumen.
Epidemiology
Spontaneous cerebral artery dissections occur primarily in middle-aged patients with a higher frequency in men, whereas traumatic dissections tend to occur at a slightly younger age. Women on average are 5 years younger than men at the time of the dissection and are more likely to have dissections in multiple vessels. The real incidence of cervicocranial dissections is hard to determine because some patients might experience minimal to no symptoms and thus remain undiagnosed. Nonetheless, the yearly detected incidence of spontaneous carotid artery dissection is thought to be around 2.6 per 100,000, whereas that of vertebral artery dissection is estimated to be at 1 to 1.5 per 100,000. Although spontaneous dissection is largely thought to be idiopathic, up to 15% of patients with spontaneous dissection are diagnosed with fibromuscular dysplasia. Other connective tissue diseases, such as Marfan syndrome, Ehlers-Danlos syndrome type IV, autosomal polycystic kidney disease, and osteogenesis imperfecta are also thought to be involved to a lesser extent.
Pathophysiology
The common pathologic finding in all dissections is a longitudinal tear within the arterial wall in the tunica media. The nature of the inciting event is debatable. The dissection is thought to be caused by a tear in the intima leading to a direct connection between intraluminal blood and the tunica media. Another hypothesis is direct extravasation of blood from the vasa vasorum into the media with subsequent proximal and distal extension of the hematoma along the vessel wall. Regardless of the inciting event, the hematoma formation may be associated with narrowing or occlusion of the arterial lumen. In addition, the dissection can potentially expose the blood stream to the prothrombotic components of the subendothelial layer, potentially leading to thrombus and distal embolic complications.
The extracranial segments of the vertebral and carotid arteries are more prone to dissections than their intracranial counterparts. The extracranial segments have greater mobility and thus have a greater risk of injury through contact with bony structures, such as the vertebral bodies for the vertebral arteries and the styloid process for the carotid arteries.
Natural history
The true natural history of cervicocranial arterial dissection is not entirely clear, but the risk of stroke would make any prospective natural history study without treatment difficult to justify. The reported mortality rate from carotid and vertebral artery dissection is approximately 5%. Although stroke might be the presenting symptom occurring at the time of dissection, it is often delayed and its onset can occur as late as 31 days after the initial injury. Ischemic manifestations typically occur within the few days after the dissection. Most dissections heal spontaneously within 3 to 6 months with medical treatment, as revealed by serial imaging. The likelihood of a dissection healing after 6 months decreases significantly. In a prospective study, however, Kremer and colleagues showed that the long-term outcome of internal carotid artery stenosis is benign, with the rate of stroke not related to the persistence of stenosis or occlusion. Nedeltchev and colleagues prospectively followed 249 consecutive patients with 268 spontaneous carotid artery dissections using ultrasound imaging at 1, 3, 6, and 12 months. Of 268 cases, 20 presented with 50% or less stenosis, 30 with 51% to 80% stenosis, 92 with 81% to 99% stenosis, and 125 with complete occlusion. The rate of complete recanalization after medical treatment was 12% at 1 month, 50% at 3 months, and 60% at 6 and 12 months. Dissecting aneurysms tend to stabilize, and they rarely grow. They can in some cases either decrease in size or even completely resolve. The risk of recurrence of spontaneous dissection within the first month is 2%, and thereafter decreases to 1% a year.
Clinical presentation
Craniocervical dissections can in some cases remain asymptomatic and undiagnosed. However, they can cause symptoms typically through 2 mechanisms:
- •
Thromboembolic: exposure of subendothelial prothrombotic components leading to platelet aggregation, with subsequent dislodgment of the thrombus and release of distal emboli.
- •
Hemodynamic: stenosis or occlusion of the true arterial lumen secondary to the expansion of the mural hematoma and to the potential formation of intraluminal thrombus, causing a decrease in distal blood flow.
In addition to these 2 mechanisms, dissections might also cause symptoms associated with subarachnoid hemorrhage (SAH) from intracranial extension of the dissection, more frequently seen with dissection in the vertebrobasilar circulation.
Dissection of the Internal Carotid Artery
The typical presentation of internal carotid artery dissection includes the triad of ipsilateral facial pain, partial Horner syndrome (oculosympathetic palsy) and subsequent ischemia. Although this presentation is encountered in fewer than a third of the cases, a high index of suspicion should be maintained in patients who present with at least 2 of these symptoms or with nonspecific focal neurologic complaints, particularly if a history of trauma can be found.
In spontaneous dissections, pain is most frequently the initial symptom. Most often it is characterized as a unilateral headache affecting the frontotemporal area, nonthrobbing in nature, with gradual onset, although it can also present as an acute-onset severe “thunderclap” headache. Orbital pain may also be present in half of the patients. In fewer than 10% of cases, pain is the only presenting symptom.
Oculosympathetic palsy is defined as ptosis with meiosis without anhidrosis. It is recognized as a typical finding of internal carotid artery dissection because the sympathetic fibers innervating the facial sweat glands follow the external carotid artery and are thus spared. Found in up to 50% of cases, any patient with a partial Horner syndrome should be considered to have an internal carotid artery dissection until proven otherwise.
In addition to pain and an incomplete Horner syndrome, an internal carotid artery dissection might also cause cranial nerve palsies in approximately 12% of patients, with a predilection for lower cranial nerves. The most commonly affected nerve is the hypoglossal (XII) because of its location in proximity to the carotid sheath. Other nerves that might be injured are the oculomotor (III), trigeminal (V), and facial nerves (VII). The clinical presentation of lower cranial nerve palsy associated with oculosympathetic impairment might therefore mimic a brain stem infarct.
Because of the potential narrowed lumen, an audible pulsatile tinnitus has been reported in up to one-fourth of cases. The bruit can be heard by the patients, and sometimes objectively presents on auscultation.
Ischemic manifestations are common in patients with carotid artery dissection, with a reported frequency of 50% to 95%. The most common mechanism of ischemia is thromboembolism. In certain cases, however, when dissection results in acute severe stenosis or occlusion, ischemic symptoms can be from hemodynamic insufficiency. Because of increased early recognition of arterial dissections as a clinical entity, added to the fact that ischemic symptoms often are a delayed manifestation of dissections, the frequency of ischemic presentations has declined over the years. Usually, ischemic stroke is preceded by warning signs, such as transient ischemic attacks and amaurosis fugax ; however, approximately 20% of patients present with an ischemic stroke without any warning signs.
Dissection of the Vertebral Artery
Patients with vertebral artery dissections typically present with pain in the posterior half of the neck, followed by ischemic manifestations in the posterior circulation. In addition to neck pain, patients often complain of headache most commonly localized in the occipital area but sometimes extending to the entire cranium or the frontal area. Patients with vertebral artery dissections also rarely present with upper extremity weakness from involvement of the C5–C6 nerve roots.
In patients with vertebral artery dissections, ischemic manifestations are very common and may present as a lateral medullary syndrome (Wallenberg syndrome), specifically if the dissection is localized in the third or fourth segment of the vertebral artery. The transient ischemic attacks or stroke may also involve other areas of the brain stem, the thalamus, the cerebral and cerebellar hemispheres, and, rarely, the spinal cord in isolation.
Diagnostic imaging
The conventional gold standard for diagnosing arterial dissection is cerebral angiography. However, with the vast improvements in magnetic resonance imaging (MRI) technology in the past decade, the use of MRI and magnetic resonance angiography (MRA) for diagnostic purposes and, more commonly, for follow-up of patients has increased significantly. The major disadvantage of magnetic resonance technology is acquisition time, which limits its use in emergency situations. Computed tomography (CT) scans, however, can be obtained quickly, can assess with high accuracy the presence of subarachnoid hemorrhage and stroke or other signs of hypoperfusion, and are widely available in most centers.
Cerebral Angiography
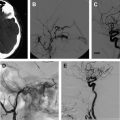
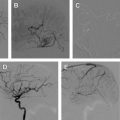
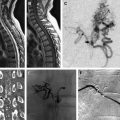
The most common finding on conventional angiography is segmental arterial stenosis or “string sign.” A “string sign” is caused by the narrowing of the vessel lumen and is not specific to dissection. It can also be seen in other conditions, such as atherosclerotic stenosis. Other findings include fusiform dilation with proximal or distal narrowing, termed the “string and pearl sign”; an intimal flap; occlusion of the vessel usually tapered to a point; and a double lumen with retention of the contrast in the false lumen well into the venous phase. The pathognomonic signs of dissection such as the double lumen and the intimal flap are seen in fewer than 10% of cases.
A typical carotid artery dissection presents with an irregular stenosis starting 2 to 3 cm above the carotid bulb and extends distally without reaching past the petrous segment where the lumen reconstitutes precipitously. Aneurysmal dilation typically occurs in the cervical portion of the artery, although these dissections can occur intracranially. Carotid arterial dissections are fusiform in nature and occur in a third of the patients.
As for vertebral artery dissections, they most commonly occur at the level of the first and second vertebrae and can extend intracranially in approximately 10% of cases. This finding occurs because, unlike the carotid artery, the vertebral artery enters the cranium through a wide foramen. The angiographic appearance is less specific than that of a carotid artery dissection.
Regardless of its location, a risk of misdiagnosis exists because the dissection could be misinterpreted on the images as either a saccular aneurysm, an atherosclerotic lesion, or a vasospasm after an SAH. With regard to atherosclerotic lesions, patients with dissection are usually younger, their lesions are typically isolated, and the stenosis is smooth. As for vasospasm, the timing of events should help clarify the diagnosis in most cases; as with dissection, the stenotic appearance is present from the start. The confusion might occur in patients who present to the emergency room in delayed fashion.
MRI
Because of its invasive nature, conventional angiography is being replaced as the standard diagnostic procedure for dissection by other noninvasive tests, such as MRI and MRA. Different magnetic resonance sequences allow for various visualizations of the arterial dissection. Although MRA would help detect any potential luminal stenosis or occlusion, T1-weighted, T2-weighted, and proton density images allow direct evaluation of the vessel wall. Although the manifestations of dissection described on conventional angiography can be seen on an MRI, the most common finding is the intramural hematoma. The hematoma appearance follows a typical pattern of blood signal change on an MRI over time caused by hemoglobin breakdown, and goes from hypointense on T1- and T2-weighted images in the hyperacute phase to a characteristic hyperintense crescent shape around a flow void on T1-weighted images in the subacute phase. Fat-saturation sequences might help differentiate small hematomas from surrounding soft tissue. Another typical finding of arterial dissection on an MRI is an increased external diameter of the vessel, with a decreased luminal diameter resulting from the stenosis.
CT
CT angiography (CTA) has been reported to show similar results to magnetic resonance techniques, with CT the preferred modality in emergency situations and trauma cases. CTA can typically show luminal stenosis and occlusion, a crescent-shaped mural thickening representing the hematoma, the intimal flap, and a dissecting aneurysm.
Ultrasound with gray-scale and Doppler color is another noninvasive imaging technique, although it should not be considered a diagnostic tool but more a screening tool because it is highly operator-dependent and has a low diagnostic performance with dissections close to the skull base. The gray-scale ultrasound might show the intimal flap, an intramural hematoma, and, in some cases, a luminal narrowing. The color Doppler typically shows a high-resistance flow.
Treatment
The major treatment objective of cervical cerebral arterial dissections is to avoid or limit neurologic deficits. Because ischemic manifestations are often delayed in their presentation, the priority in managing these patients is determining whether they can be managed medically or whether surgical or endovascular intervention is needed. In addition, although it is widely accepted that the most likely pathophysiologic mechanism of ischemic complications is embolic, hemodynamic compromise can play a role in certain cases, particularly in patients with acute arterial occlusion. These patients might benefit from more-aggressive approaches. Treatment options include thrombolysis, anticoagulation, endovascular interventions, and surgical intervention. Unfortunately, no randomized trial has compared the various treatment options and their efficacy.
Medical Management
Thrombolysis
Little evidence in the literature shows thrombolysis in the setting of cervical cerebral arterial dissection. However, the use of intravenous or intra-arterial recombinant tissue plasminogen activator or urokinase could be warranted in patients in whom ischemic symptoms stem from an acute blockage of blood flow. Potential complications of thrombolysis include an increase in size of mural thrombus with worsening of the luminal stenosis, or even mobilization of the mural thrombus with distal embolization. In addition, thrombolysis could cause an SAH because of leakage, with the potential subsequent development of a pseudoaneurysm. The risk of developing these complications is difficult to assess because of the lack of data in the literature. Georgiadis and colleagues reviewed 33 patients treated with intravenous thrombolysis for acute stroke because of spontaneous cervical carotid artery dissection and found no new or worsening of local signs, SAH, or pseudoaneurysms. In addition, a meta-analysis of individual patient data of 180 patients from 14 retrospective series and 22 case reports showed that thrombolysis had a similar safety and outcome profile in patients with stroke caused by cervical artery dissections and those with all causes of stroke. Thrombolysis seems to be a safe treatment modality, but further studies are warranted to evaluate its efficacy.
Antithrombotic therapy
In 2007, the Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) study group published a summary of the pathophysiologic and clinical considerations regarding the use of antithrombotic agents in arterial dissection, and performed a systematic meta-analysis of the published data comparing their efficacy.
Several arguments support the hypothesis of an embolic mechanism behind the ischemic manifestations of dissections, and thus are in favor of antithrombotic therapy. The infarct pattern on brain imaging, the presence of distal branch occlusion, and the observation of microemboli all favor a thromboembolic mechanism. Transcranial Doppler studies in extracranial vertebral and carotid artery dissections revealed findings suggesting emboli in the posterior and middle cerebral artery, respectively. Also, theoretically, because most dissections heal and recanalize spontaneously, the intramural thrombus might be mobilized and throw clots into the bloodstream, thereby causing infarctions.
However, the same arguments that could potentially complicate the use of thrombolytics in arterial dissection patients apply to anticoagulation, including increase of intramural thrombus with local compression symptoms (cranial nerve palsies, Horner syndrome), and hemodynamic-induced infarcts. Unlike with thrombolytics, delayed occlusion of the internal carotid artery has been reported with the use of heparin. Finally, the risk of hemorrhagic transformation in patients with severe ischemic strokes should always be considered before an anticoagulation therapy is administered.
Because no randomized trials have been performed, no high-level recommendation can be produced. Nonetheless, it is widely thought that, unless specific contraindications are present, antithrombotic therapy should be started in the acute phase of a cervical cerebral arterial dissection.
Antithrombotic treatment consists of either anticoagulation with intravenous heparin followed by warfarin, or antiplatelet therapy with aspirin. Currently no clear evidence supports the use of one treatment over the other, although anticoagulation is typically preferred and more widely used. Anticoagulation might be preferred in patients with severe stenosis, occlusion, or pseudoaneurysm, based on the hypothesis that it is more effective in preventing a thromboembolic complication. Applying the superiority of anticoagulation to antiplatelet therapy in preventing secondary stroke in cases involving cardioembolic causes as an argument in favor of anticoagulation in preventing cervical artery dissection is questionable. Antiplatelet use might be preferable for patients with a poor prognosis or those with large infarcts. The CADISP study group performed a meta-analysis comparing the 2 treatment modalities. Their analysis included 26 studies and 327 patients, and showed no significant differences between the treatment modalities in terms of “death from all causes” and “death and disability.” A Cochrane systematic meta-analysis of nonrandomized studies showed similar results. However, both analyses cite a lack of data from randomized trials, which therefore limits the potential for a real evaluation of their efficacy, let alone a true comparison between anticoagulation and antiplatelet therapies in cervical cerebral dissections.
Duration of treatment is open to debate. Typically, because dissections heal within 6 months of onset and rarely recur after, treatment should be continued for 3 to 6 months. Imaging findings have been suggested as potential guidelines for continued anticoagulation of lack thereof, but this method has not been proven.
Endovascular Management
Indications
Endovascular interventions should not typically be considered as first-line treatment for spontaneous cervical cerebral dissections because of the low recurrence rate of dissections combined with the risk of iatrogenic complications of invasive interventions. However, certain clinical scenarios exist in which an endovascular or a surgical intervention is warranted. First and foremost, patients presenting with an SAH should be considered surgical or endovascular emergencies, particularly those with intracranial dissections in the posterior circulation. These patients should first undergo a diagnostic angiogram to assess for a direct cause of the hemorrhage, such as a pseudoaneurysm. The diagnostic angiogram would also study the collateral circulation, the length of the dissected segment, and vertebral dominance. Intracranial pseudoaneurysms have a reported mortality rate as high as 83% and a rerupture rate of approximately 70% within the first 24 hours of presentation. Occlusion of the entire dissected segment with or without flow replacement is the treatment option associated with the lowest risk of rehemorrhage. Although this could be achieved through proximal occlusion, trapping of the dissected segment might be a better option to avoid filling of the occluded segment through a retrograde flow from the contralateral vertebral artery. The procedure can be performed endovascularly, although in certain cases an open surgical approach might be preferable to visualize and preserve the posteroinferior cerebellar artery (PICA) and brainstem perforators. In cases of ruptured vertebral artery dissection that extends to the basilar artery, treatment options are limited. These lesions are notoriously difficult to treat. If the patient passes the balloon test, occlusion and then a proximal occlusion of the basilar artery or bilateral occlusion of the vertebral arteries might be the best option. Otherwise, a stent-in-stent technique can be attempted with potential stent coiling of the pseudoaneurysm.
Another clinical vignette in which endovascular treatment is indicated in the acute setting is when dissection results in acute arterial occlusion. In these cases, hemodynamic compromise, and not thromboembolism, is the mechanism behind ischemia of cerebral tissue. In these patients, attempts can be consistent to restore flow. First, high-volume fluid resuscitation should be started to reach a normotensive state with a likely benefit of a slight hypertensive state. An arterial line and a Foley catheter could also help strictly monitor the patient’s volemic state, the objective being a euvolemic or a slightly hypervolemic state. Once the patient is stabilized, an intervention to restore normal flow should be considered.
Other than these 2 indications, endovascular therapy should be considered if medical management fails or the patient has a contraindication to antithrombotic agents. Although no textbook definition of failure of medical therapy exists, it is generally agreed that progression of neurologic symptoms and new ischemic events despite adequate antithrombotic therapy define failure of medical therapy. Whether pseudoaneurysm enlargement can be considered failure of medical therapy is controversial.
Technical nuances
Preoperative assessment of images is critical. Length of the dissected segment, associated pseudoaneurysm, and location of important arteries and perforators should be assessed before the patient is taken to the interventional suite. Furthermore, understanding of the clinical presentation is important and might help determine the antithrombotic strategy after intervention. Because guidewires and catheters are thrombogenic, efforts should be made to perform the procedure as efficiently and quickly as possible without compromising patient safety. To avoid iatrogenic complications, including thromboemboli, every effort should be made to minimize intravascular manipulation. A microcatheter should be passed atraumatically over the dissection. At that point, contrast material should be injected to ensure that the catheter is actually in the true lumen. Once confirmed, an exchange wire should be left beyond the dissected segment. Ideally, wire access should not be lost until stenting is completed. Different types of stents can be used. Balloon-expandable stents exert a high radial force on the vessel wall and have an increased metal-to-artery wall surface. They can therefore be used to great advantage in patients with extracranial artery dissections. Their high radial force reapposes the intimal flap and attaches the thrombus on the vessel wall. Their limitation is that they are rigid and cannot be guided into the smaller, more tortuous intracranial vasculature. Furthermore, their lack of flexibility limits their use in the skull base, where flexion and extension of the neck might kink and thus occlude the stent. Self-expandable stents offer more flexibility and a lower radial force, and are thus better suited for intracranial placement. They can be more easily guided into the intracranial vessels and resist compression after placement.
Finally, unlike stenting for carotid artery stenosis, it is generally agreed that the risk of worsening the dissection with the use of distal embolic protection devices outweighs any benefits that might stem from their deployment. Investigators have hypothesized that perhaps proximal protection devices might prove beneficial in this patient population.
Surgical Management
Endovascular management has largely replaced surgical treatment of arterial dissections. It is still useful in patients who present with SAH and in whom proximal occlusion or trapping of the dissected segment is indicated. Surgery offers the benefit of better visualization of the perforators and PICA. Other surgical procedures indicated for managing dissection include extracranial-intracranial bypass as flow replacement for cases treated with trapping, and thromboendarterectomy for carotid dissections.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree