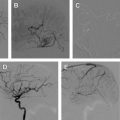
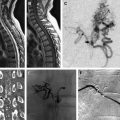
Dural arteriovenous fistulas (DAVFs) are arteriovenous shunts from a dural arterial supply to a dural venous channel, typically supplied by pachymeningeal arteries and located near a major venous sinus. Pial arteriovenous fistulas (PAVFs) are composed of one or more arterial feeders draining into a single vein in the absence of an intervening nidus. Fistulas manifesting features of high risk for rupture should be treated aggressively, the spectrum of treatment varies from endovascular, surgical resection, and stereotactic radiosurgery. This article describes the natural history, clinical presentation, and treatment of dural and pial fistulas, with emphasis on endovascular treatment.
Key points
- •
Dural arteriovenous fistulas (DAVFs) are arteriovenous shunts from a dural arterial supply to a dural venous channel, typically supplied by pachymeningeal arteries and located near a major venous sinus.
- •
DVAFs with retrograde venous drainage can result in hemorrhage or cause decreased regional cerebral blood flow in cortical regions involved.
- •
DAVFs can be treated with surgery, endovascular embolization (transarterial or transvenous approach), and radiosurgery.
- •
Elimination of the retrograde cortical venous drainage is the goal of any type of treatment for DAVFs.
- •
Pial Arteriovenous fistulas (PAVFs) are direct fistulas from an intracranial arterial feeder into a single venous channel and typically have high risk of intracranial hemorrhage and death if left untreated.
- •
PAVFs can be treated with surgery or endovascular embolization. Radiosurgery is not used because of the latent effect and difficulties in targeting the fistula.
Dural arteriovenous fistulas
Dural arteriovenous fistulas (DAVFs) are arteriovenous shunts from a dural arterial supply to a dural venous channel, typically supplied by pachymeningeal arteries and located near a major venous sinus. The etiology of these lesions is not fully understood; some are congenital, and others are acquired. DAVFs in the pediatric population are associated with structural venous abnormalities, but most DAVFs are thought to be acquired. The development of venous obstruction and hypertension with aberrant angiogenesis can contribute to the pathogenesis of these lesions. This altered angiogenesis occurs within the dura following an inciting event such as trauma, surgery, chronic infection, or sinus thrombosis. As microshunts proliferate in association with venous hypertension, these mature into clinically significant DAVF. The degree of progression or involution determines the significance of the abnormality. This can then result in hemorrhage or other focal manifestations including hemodynamic insufficiency. DAVFs can also cause decreased regional cerebral blood flow in cortical regions where there is retrograde venous drainage.
At the same time, some cases of DAVF have no clear inciting event or are at a site that is clearly distinct from the presumed inciting event. It is thought that the development of a DAVF in these settings requires a common unclear mechanism as well as a possible anatomic or genetic predisposition.
DAVFs have been reported in all age groups, but mainly in the fifth and sixth decades of life. The estimated incidence of DAVFs is 0.17 cases per 100,000 population, and they are one-fifth as common as arteriovenous malformations (AVMs). They represent 10% to 15% of all intracranial vascular malformations, with a higher incidence in women. A female-to-male ratio of 2:1 exists in certain anatomic sites such as the cavernous and transverse–sigmoid sinuses. DAVFs are usually solitary, although in 5% of cases, multiple lesions have been described. The goal of this article is to describe the natural history, clinical presentation, and treatment of dural and pial fistulas with emphasis on the endovascular treatment.
Natural History
An established DAVF may follow 1 of several unpredictable natural courses. Some lesions remain asymptomatic or maintain stable clinical symptomatology and angiographic features over many years. Others undergo spontaneous regression, involution, and resolution with stabilization or improvement of neurologic symptoms. Features that may predispose to such spontaneous involution are not known. DAVFs in the region of the cavernous sinus are particularly prone to this phenomenon, with as many as 40% of reported cases having undergone spontaneous involution.
In contrast, some DAVFs may demonstrate an increase in size from either arterial or venous enlargement or even de novo development of a DAVF. Pachymeningeal arterial feeders may be progressively recruited causing enlargement of the nidus due to unknown mechanisms. This results in hypertrophy of dural arteries and the reappearance of involuted embryonic arteries that may not normally be visible in the adult dura mater. In some DAVFs there is also progression on the venous side. Progressive arterialization of the pathologic dural leaflets results in hypertension in adjacent leptomeningeal venous channels, leading to retrograde leptomeningeal venous drainage. Under arterialized pressures these channels may become tortuous and become varices or aneurysms. The catastrophic consequence that ensues is a cerebral hemorrhage from retrograde cortical venous drainage (CVD).
Clinical Presentation and Assessment
Clinical manifestations of DAVFs are highly variable and are related primarily to the location of the fistula as well as retrograde CVD. These range from minor symptoms to intracranial hemorrhage. The vast majority of symptoms can be attributed to the anatomy of the DAVF.
Patients’ symptoms may be sudden or slowly progressive. The degree and type are determined by venous topography, venous flow pattern, and the capacity of surrounding compensatory venous drainage. The most serious neurologic sequelae from DAVFs are associated with retrograde CVD, leading to a propensity to rupture. Focal neurologic deficits likely result from venous hypertension and intracranial hemorrhage from rupture of arterialized leptomeningeal veins.
There are a wide variety of nonhemorrhagic symptoms with which DAVF can present. Relatively benign symptoms such as pain, tinnitus, or bruit are related to arteriovenous shunting and flow within the DAVF. Pulsatile tinnitus or other auditory symptoms may occur with or without pain. These symptoms are likely related to high flow through dural vascular channels at the base of the skull. Other more painful complaints may be related to orbital congestion, stretching of dural leaflets by engorged vascular channels, or to direct compression of the trigeminal nerve by arterialized venous structures near the petrous apex.
Various neuro-ophthalmologic manifestations of DAVFs include visual and gaze abnormalities caused by venous hypertension. Orbital or ocular venous hypertension with resulting orbital crowding, venous stasis retinopathy, and glaucoma can also be seen.
Other intracranial DAVFs may present with symptoms of increased intracranial pressure (ICP) or a poorly defined headache. While the headaches are nonspecific, there does appear to be an association with the dysplastic changes in meningeal vessels that are often present in DAVFs. There are also a wide spectrum of neurologic symptoms including seizure, hearing loss, cranial nerve palsy, papilledema, vision changes, and motor/sensory deficits that can be seen with intracranial DAVF.
DAVFs may also result in altered cerebrospinal (CSF) flow. Dilated venous structures may act as mass lesions, obstructing the CSF circulation and causing hydrocephalus. In other cases, dural venous hypertension may result in decreased absorption of CSF with secondary intracranial hypertension and papilledema. This latter complication appears to be more common in high-flow lesions draining into large dural venous sinuses in the setting of concomitant sinus outflow obstruction.
Certain clinical presentations are seen with DAVFs in specific locations. DAVFs in the region of the transverse or sigmoid sinus, or near the cavernous sinus, often drain into the associated venous sinuses and may cause a variety of clinical manifestations due to increased flow or local venous engorgement. High-flow lesions in the region of the transverse sigmoid sinus junction often result in pulsatile tinnutus, headache, and bruit. This phenomenon does not lead to intracranial hemorrhage unless there is associated retrograde CVD. Lesions at the anterior cranial fossa or the tentorial incisura rarely drain into a patent dural venous sinus and are more frequently associated with leptomeningeal venous drainage. They are more likely to cause serious clinical sequelae from venous hypertension and hemorrhage.
Imaging Studies and Classification
Dilated or thrombosed venous structures on head computed tomography (CT) and brain magnetic resonance imaging (MRI) may suggest the presence of a DAVF. However, these routine studies are frequently equivocal and do not provide information about the anatomy of the fistula. CT angiography and magnetic resonance angiography (MRA) are important noninvasive diagnostic studies that provide not only anatomic details but may be coupled with perfusion studies as well to evaluate the effect of a DAVF on regional blood flow. Angiography is needed for definitive diagnosis and pretreatment analysis of intracranial DAVFs. Projections usually include the external and internal carotid arteries bilaterally and the vertebral arteries. A thorough study of the arterial supply, anastomoses, and venous anatomy is performed before the use of embolic materials.
Classification of DAVFs has evolved over time to be useful in guiding therapeutic intervention. Initial attempts were simplistic, emphasizing the anatomic location, but lacked meaningful information in regard to predicting the nature or outcome of the abnormality. Subsequent systems incorporated information from diagnostic angiography.
Perhaps 1 of the most well recognized classification schemes specific to DAVFs is that developed by Djindjian and colleagues. This system categorizes a lesion into 1 of 4 types. Type I DAVFs are characterized by normal anterograde drainage into a venous sinus or meningeal vein; type II lesions drain into a sinus, with reflux into adjacent sinuses or cortical veins. Type III DAVFs drain directly into cortical veins with resultant retrograde flow into the cerebral venous compartment, and type IV DAVFs have drainage directly into a venous pouch (venous lake or venous ectasia). Djindjian and colleagues concluded from their study that type I DAVFs were benign, with each sequential type having more aggressive characteristics. Since the introduction of the Djindjian classification, other studies have been published in the literature attempting to correlate certain features of the DAVF with the likelihood of hemorrhage or other neurologic complications.
With the advent of more effective endovascular techniques, a means of predicting lesion risk and management options emerged. Cognard and colleagues developed a classification system derived from a modified version of the Djindjian classification. They defined 5 types of DAVFs that are based on the pattern of venous outflow. Type I DAVFs were characterized by normal antegrade flow into the affected dural sinus. Type II lesions were associated with an abnormal direction of venous drainage within the affected dural sinus. These lesions could be further categorized into 3 subtypes: type IIa, lesions with retrograde flow exclusively into a sinus or sinuses; type IIb, lesions with retrograde venous drainage into the cortical veins only; and type II a + b, lesions with retrograde drainage into sinuses and cortical veins. Type III DAVFs drained directly into a cortical vein or veins without venous ectasia, whereas type IV DAVFs had drainage into cortical veins with the critical component of venous ectasia greater than 5 mm in diameter and 3 times larger than the diameter of the draining vein. A DAVF was considered to be type V when drainage was into spinal perimedullary veins. Correlation with their clinical data yielded the following conclusions.
Type I DAVFs are considered benign, and treatment is usually not necessary, except possibly for palliation of symptoms.
Type IIa lesions are best treated with arterial embolization.
Type IIb and IIa + b lesions usually require both transarterial and transvenous embolization for effective obliteration.
For types III to V, transarterial embolization and occasionally transvenous embolization aimed at complete occlusion of the fistula are necessary and often will need to be combined with surgical techniques to eradicate the threatening cortical venous drainage.
Borden and colleagues also proposed a classification system emphasizing venous anatomy with 3 categories. Type I DAVFs drain directly into dural venous sinuses or pachymeningeal veins. Type II DAVFs drain into dural sinuses or pachymeningeal veins but also have retrograde drainage into subarachnoid (leptomeningeal) veins. Type III DAVFs drain solely into subarachnoid veins and do not have dural sinus or meningeal venous drainage. The validity of both the Cognard and Borden classification systems was confirmed in 102 intracranial DAVFs in 98 patients ( Table 1 ).
| Type | Djindjian | Cognard | Borden |
|---|---|---|---|
| I | Normal antegrade flow into dural sinus | Normal antegrade flow into dural sinus | Drains directly into venous sinus or meningeal vein |
| II | Drainage into venous sinus with reflux into adjacent sinus or cortical vein |
| Drains into dural sinus or meningeal veins with retrograde drainage into subarachnoid veins |
| III | Drainage into cortical veins with retrograde flow | Direct drainage into cortical veins without venous ectasia | Drains into subarachnoid veins without dural sinus or meningeal involvement |
| IV | Drainage into venous pouch (lake) | Direct drainage into cortical veins with venous ectasia >5 mm and 3× larger than diameter of draining vein | |
| V | Drainage to spinal perimedullary veins |
Regardless of lesion location, clinical presentation, or other symptomatology, the most important factor determining the propensity of a lesion to have an aggressive clinical course appears to be the presence of leptomeningeal venous drainage. Lesions that drain into a large patent venous sinus may have various clinical associations but are less likely to bleed or cause focal neurologic deficits unless associated with retrograde leptomeningeal venous drainage. Lesions without drainage into a patent dural venous sinus are more frequently associated with leptomeningeal venous drainage and prone to serious clinical sequelae such as an intracerebral hemorrhage. The risk of hemorrhage appears to be related directly to the presence of tortuous and aneurysmal leptomeningeal arterialized veins in association with DAVFs.
Treatment and Follow-up
A DAVF may rarely be discovered on imaging studies or digital subtraction angiography performed for other indications. Incidental lesions must be carefully assessed for features predisposing to aggressive clinical behavior. Complete angiographic evaluation is indicated in every case of suspected DAVF unless the patient is a poor candidate for therapeutic intervention or refuses invasive diagnostic studies. Lesions should be evaluated specifically for the presence of leptomeningeal venous drainage, varices (aneurysmal changes in the venous circulation), and venous ectasia. In the absence of these features, the lesion should be followed expectantly. There is no evidence demonstrating significant benefits to prophylactic treatment of unruptured DAVFs that are not associated with leptomeningeal cortical venous drainage. Expectant follow-up of these lesions should include serial MRI for any evidence of changes in the DAVF anatomy. Angiographic re-examination of the lesion every few years should be considered, especially for DAVFs at the anterior cranial fossa or the tentorial incisura, which commonly harbor leptomeningeal venous drainage.
Definitive prophylactic treatment should be strongly considered for asymptomatic and incidentally discovered DAVFs with leptomeningeal venous drainage. The patient should be given the option of open surgical, radiosurgical, or endovascular interventions as may be appropriate for the specific lesion type and location. If treatment does not succeed at totally eliminating leptomeningeal venous drainage, then further definitive therapy or close follow-up of the lesion is indicated. Anticoagulation is contraindicated in the setting of DAVFs with leptomeningeal venous drainage.
Definitive intervention for DAVFs that behaved aggressively in the past warrants serious thought. The morbidity of a first hemorrhage with DAVFs is substantial, and many patients do not survive or recover to a condition suitable for therapeutic intervention. However, there are numerous documented cases of progression of focal neurologic symptoms resulting in death or major disability unless the DAVF is obliterated. Lesions that have hemorrhaged or caused focal neurologic symptoms due to venous hypertension without retrograde CVD should still undergo definitive treatment in those patients who are clinically stable. Palliative therapy is often inadequate in this setting. Those patients with retrograde CVD with leptomeningeal spread presenting with hemorrhage clearly need an intervention.
On the other hand, lesions that present with pain or pulsatile tinnitus are evaluated and treated in the same way as incidental lesions. Nonspecific measures aimed at resolving the symptoms are often sufficient. Palliative treatment of the DAVF may be considered for the control of symptomatology. Rarely is definitive treatment indicated solely for pain or pulsatile tinnitus.
Endovascular Neurosurgical Techniques
Transarterial embolization (TAE) has been widely used in the treatment of DAVFs. The use of flow-guided catheter technology and increased experience with particle and glue embolization, as well as detachable coils have greatly improved the safety and efficacy of this method. However, TAE rarely succeeds in totally eliminating a DAVF except in rare instances of limited fistulae with a small number of accessible feeders. More commonly, DAVFs involve a multitude of feeders, which often arise as multiple, small tributaries from major cerebral arteries that are not amenable to TAE. While TAE may obliterate the filling of the lesion after 1 injection, the DAVF often continues to draw feeders from other sources and will reappear on subsequent angiography. DAVFs that are partially treated with TAE may later recur and result in hemorrhage.
TAE can be effective in palliating disabling symptoms even without completely curing the DAVF. Symptomatic palliation may be accomplished by TAE of feeders to the DAVF, although such an intervention is not without risk and not always successful in eliminating the DAVF. Arterial embolization may give a false sense of security that the lesion was treated, while the DAVF may progress to acquire more aggressive features including leptomeningeal venous drainage (even in the absence of recurrent symptoms). DAVFs that are followed expectantly or treated palliatively should be monitored closely with serial diagnostic imaging. TAE also plays an important role in decreasing flow through DAVFs before surgical intervention, transvenous obliteration, or radiosurgery. This preparatory use of TAE has greatly enhanced the safety and efficacy of other treatment measures.
Noninvasive imaging methods, including MRI and MRA, may be used for interval studies, although these modalities may miss subtle development of leptomeningeal venous drainage. Depending on the clinical situation and the particular lesion, serial magnetic resonance studies may be performed on a yearly basis, with formal angiography every few years or sooner if symptoms change, or if there is a suggestion of new leptomeningeal venous drainage on MRI.
Transvenous embolization (TVE) of DAVFs has recently been used with good results. This modality aims at the thrombosis of the venous side of the lesion, often including the obliteration of the adjacent dural venous sinus. Occlusion of the venous side of DAVFs is usually well tolerated if the pathologic dural sinus is arterialized and does not serve as a site of drainage of normal circulation. Instead, the pathologic dural segment is often associated with harmful retrograde leptomeningeal venous drainage, and these channels are secondarily obliterated with thrombosis of the venous side of DAVFs. This strategy has been used most successfully in the treatment of DAVFs with accessible venous drainage. TVE is particularly effective in the treatment of cavernous sinus DAVFs (via the inferior petrosal sinus), although these lesions frequently do not require any therapeutic intervention because of their benign clinical symptomatology and tendency toward spontaneous regression.
TVE has also been used in cases of transverse sigmoid sinus DAVFs, and may be substantially safer than open surgical approaches to these lesions. However, there may be no accessible transvenous route for many DAVFs, including tentorial incisura DAVFs and anterior cranial fossa DAVFs, which frequently behave aggressively. Transvenous obliteration may occasionally be performed after open surgical exposure, through puncture of the dural venous sinus or the arterialized venous varix with the injection of coils or glue. Rarely, TVE may result in propagating venous thrombosis or altered hemodynamic patterns with paradoxic clinical deterioration or hemorrhage.
Various embolic materials have been used, including particles, liquid silicone, ethyl alcohol, platinum microcoils, and cyanoacrylates for endovascular therapy. N-butyl cyanoacrylate (NBCA) has a fast polymerization rate and binding properties, making it somewhat difficult to use. Both preparation and delivery of NBCA require an experienced user. NBCA is injected until it reaches the proximal draining vein, and then the microcatheter is then removed promptly to prevent its adhesion to the NBCA; the D5W push technique has been used to try to push, then glue distally in the fistula to the venous side, and the use of glacial acetic acid has been also described to delay the polymerization of the glue, enabling the glue to travel distally.
Onyx liquid embolic system is an ethylene vinyl alcohol polymer dissolved in dimethyl sulfoxide (DMSO). Injection of onyx in blood results in solvent diffusion, allowing the polymer to precipitate and mechanically occlude the vessel. Injection of larger amounts of onyx can result in filling of the fistulous network and allow for reflux into other arterial feeders. At the same time, there have been theories that onyx can leave microchannels within the cast that allow small amounts of flow through the fistula, resulting in recurrence of the fistula or possibly residual fistula despite angiographic embolization.
In addition, onyx has been known to cause significant inflammation within the vasculature. This can be seen in the treatment of cavernous carotid fistulas in which the surrounding cranial nerves are often irritated, resulting in various cranial nerve palsies. The significance of this inflammation in other areas remains to be seen. Others report that theoretically there should be less inflammation than NBCA, since there is no protein denaturation, which has been demonstrated in animal models. Regardless, onyx remains an important and effective modality in the armamentarium against fistulas and other vascular anomalies.
Occasionally a DAVF will recur adjacent to an endovascularly occluded venous sinus, and this could represent reconstitution of arteriovenous channels within the walls of the occluded sinus, or in the organized thrombus within the sinus channel. These cases are amenable to surgical excision of the segment of occluded sinus with disconnection of associated arterialized leptomeningeal veins.
The Jefferson Hospital for Neuroscience Experience
Methods
Thirty-nine patients (22 men and 17 women) underwent endovascular treatment of DAVFs at the authors’ institution from 2001 to 2009 ( Table 2 ). Ages ranged from 39 to 71 (mean 48). Seventy-nine percent of patients had retrograde CVD. Upon completion of diagnostic angiography, transarterial embolization was attempted first with either NBCA or onyx. The number of arterial embolizations and need for transvenous embolization, open surgery, or radiosurgery were assessed. Normalization of retrograde CVD was also assessed. Finally, postoperative complications were addressed.
| Characteristics | n (%) |
|---|---|
| Time period | 2001–2009 |
| Number of patients | 39 |
| Age range | 39–71 |
| Median age, y | 48 |
| Number of treatments | |
| Onyx | 12 |
| NBCA | 11 |
| Coils | 5 |
| Craniotomy | 2 |
| Radiosurgery | 7 |
| Median volume, cc | 2.5 |
| Median dose, Gy | 22 |
| Cortical venous drainage | 18 |
| Complete treatment | 28 (71) |
| Endovascular alone | 21 |
| Endovascular followed by resection | 7 |
| Transvenous approach | 5 |
| Transarterial approach | 25 |
Results
Obliteration of DAVF
The average number of embolizations in all patients was 2.1. Patients were considered completely treated when there was a greater than 95% reduction in DAVF flow based on the angiographer’s radiographic assessment. Seventy-one percent (28 of 39) of patients had complete treatment of the fistula: 21 by purely endovascular treatment and 7 with endovascular therapy followed by craniotomy.
Of the 11 patients who did not have complete treatment of the fistula, 7 (64%) had at least 90% obliteration with only 1 feeding pedicle remaining. Three of those with incomplete treatment (but absent CVD) underwent radiosurgery as the final approach for treatment. The average dose for treatment of the DAVF was 22 Gy in single fraction; follow-up in this subgroup is ongoing.
Use of onyx in DAVF embolization
Forty treatments were made for 12 patients ( Table 3 ). There were 3.33 treatments per patient, with a complete obliteration rate of 75% at the end of the follow-up period. The cessation of CVD was seen in 85% of patients at end of follow-up for patients with onyx treatment.
| n (%) | |
|---|---|
| Treatments | 40 |
| Number of patients | 12 |
| Complete obliteration of fistula | 8 (75) |
| Median treatments per patient | 3.33 |
| Morbidity | 3 (7.5) |
| Mortality | 0 |
| Reversal of cortical venous drainage | 30 (85) |
Normalization of retrograde CVD
Of those patients with retrograde CVD, 87% (26 of 30) had resolution with treatment. Of these 26 patients, 69% (18 of 26) patients had obliteration by endovascular means alone, while the rest required open surgical clipping of the fistula following embolization.
In 5 of the 18 patients who had CVD treated via embolization, success was only obtained when a transvenous approach was performed, due to difficulty with catheterization of feeding vessels. Three of the 5 patients received coils in addition to onyx for transvenous embolization.
Postoperative complications
Epidural infection after craniotomy, postembolization intracranial hemorrhage not requiring surgery, and need for femoral artery repair after embolization were all encountered ( Table 4 ). All complications were diagnosed and treated expeditiously.
| Complication | n (%) |
|---|---|
| Stroke | 1 |
| Femoral dissection | 1 |
| Retroperitoneal hematoma | 0 |
| Postembolization-associated hemorrhage | 1 |
| Renal failure | 0 |
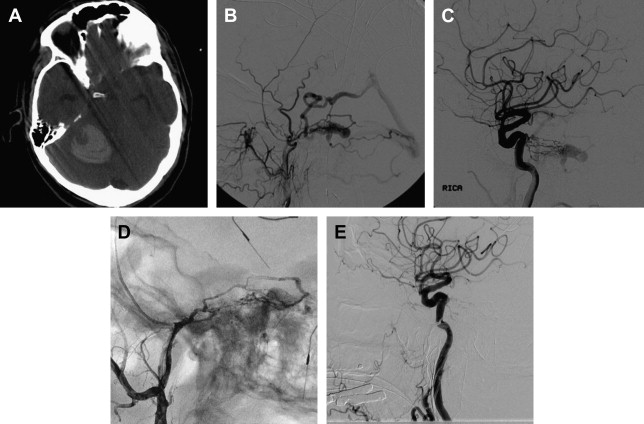
Fig. 1 shows a case illustration of a DAVF treated with onyx.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree