Arterial Spin Labeling in Arteriovenous Malformations
Ronald L. Wolf
Jeffrey M. Pollock
Arteriovenous malformation (AVM) and arteriovenous fistula (AVF) represent vascular malformations that have arterial-to-venous connections bypassing a normal capillary bed. The most common presenting event is intracranial hemorrhage (40% to 70% of patients), and other clinical presentations include headache, seizures, tinnitus, and even heart failure in newborns. Digital subtraction angiography (DSA) is the current gold standard for evaluation of vascular malformations and can be combined with endovascular treatment, alone or in combination with other treatment strategies including stereotactic radiosurgery or surgery. Risks involved in DSA with or without endovascular therapy include vascular injury, thromboembolic events, intracranial hemorrhage, allergic reactions, and ionizing radiation exposure, and this is compounded when multiple diagnostic or embolization sessions are needed for treatment or follow up. DSA is limited in quantitative measurement of arteriovenous (AV) shunt or cerebral blood flow (CBF).
Although there are noninvasive alternatives for evaluation of vascular malformations like computed tomography (CT) angiography and magnetic resonance angiography (MRA), these are in general inadequate for detailed evaluation of the complex anatomy and hemodynamic effects including impact on CBF; however, innovations in both CT angiography and MRA are beginning to provide access to more comprehensive four-dimensional analysis of vascular malformations.1,2 CT and MR techniques for assessing the hemodynamic effects of these lesions have also been described. Arterial spin labeled (ASL) perfusion magnetic resonance imaging (MRI) strategies for evaluation of intracranial vascular malformations are the subject of this chapter.
Clinical Background and Current Imaging Practices
Vascular Malformations
Intracranial vascular malformations with AV shunting include AVM, where blood flow passes from arterial to venous system through a nidus or low-resistance tangle of vessels, and AVF, where blood flow passes directly from artery to vein in one or more locations. Options for treatment include surgery, endovascular embolization, or stereotactic radiosurgery. Surgical treatment with or without embolization can be definitive, but there are associated risks including hemorrhage, infarct, and potential deficits related to location of lesion in proximity to eloquent brain. Stereotactic radiosurgery with or without embolization can be used as well but cannot be used for larger AVMs nor is it used for AVFs, and there is a delay in response over 1 to 3 years. The likelihood of successful surgical outcome for AVM can be estimated based on size, drainage pattern, and anatomic location (Spetzler-Martin grading system).3
Angiographic Evaluation of Vascular Malformations
Digital Subtraction Angiography
For assessment of AVM, at minimum a technique must show feeding arteries, size of nidus, internal morphology, or more specifically aneurysms, and pattern of venous drainage. For this purpose, DSA remains the reference standard with unsurpassed temporal and spatial resolution, although there are some risks, as noted previously. In routine implementation, this is a two-dimensional projection technique, but rotational angiography can provide three-dimension volumetric reconstructions and is now used routinely. Although visual estimates of degree of shunt or flow rate in an AVM are possible and objective indirect measures such as venous arrival time are possible, DSA is not capable of accurately quantifying AV shunt or CBF in adjacent brain. Also, evaluation of proximity to eloquent structures is better accomplished with MRI and to a lesser extent CT.
Magnetic Resonance and Computed Tomography Angiography
Routine time-of-flight MRA is often initially employed in AVM or AVF assessment and can provide useful diagnostic information,4 but it does not provide an adequate assessment of vascular architecture or functional evaluation of hemodynamics. Phase-contrast MRA techniques are likewise limited in delineation of the vascular architecture but do provide for objective functional assessment in that flow rates, at least in supplying (or draining) vessels, can be quantified5,6; however, this can be time-consuming when multiple vessels need to be interrogated.
Contrast-enhanced techniques have improved to the point where spatial resolution approaches that of DSA, and temporal resolution is also improving to where subsecond acquisitions are now routinely available. Optimal spatial and temporal resolution at the same time and in
three dimensions are difficult to achieve7,8,9; however, MRA and CT angiographic techniques have improved to the point where detailed and fairly accurate noninvasive preoperative evaluation is now possible. For CT angiography, the advent of scanners with a large number of detectors sufficient for scanning all or nearly all of the brain in one rotation has provided for relatively efficient CT DSA, from which perfusion data can also be extracted.10 MR DSA (four-dimensional MRA) techniques are also available and have been employed for relatively accurate grading of AVMs.2 Selective evaluation of individual vessels is not routinely available for contrast-enhanced techniques, but four-dimensional phase-contrast MRA approaches may ultimately obviate the time considerations for flow quantification in large vessel feeders.11
three dimensions are difficult to achieve7,8,9; however, MRA and CT angiographic techniques have improved to the point where detailed and fairly accurate noninvasive preoperative evaluation is now possible. For CT angiography, the advent of scanners with a large number of detectors sufficient for scanning all or nearly all of the brain in one rotation has provided for relatively efficient CT DSA, from which perfusion data can also be extracted.10 MR DSA (four-dimensional MRA) techniques are also available and have been employed for relatively accurate grading of AVMs.2 Selective evaluation of individual vessels is not routinely available for contrast-enhanced techniques, but four-dimensional phase-contrast MRA approaches may ultimately obviate the time considerations for flow quantification in large vessel feeders.11
Another advantage with MR and CT techniques is the ability to evaluate the brain parenchyma, and with this there is the potential for assessing effects on adjacent (and distant) brain resulting from the hemodynamic disturbances created by vascular malformations. Techniques such as functional MRI and perfusion imaging allow for assessment of function and physiology. These can be combined with conventional structural and angiographic imaging to complement traditional approaches and potentially provide additional useful information for prognosis, treatment planning, and evaluation of treatment efficacy.
Perfusion Techniques
Clinical perfusion imaging techniques use tracers that fall into two basic categories: diffusible and nondiffusible. Nondiffusible tracers are confined to vessels (as long as the blood–brain barrier is intact), with current techniques including primarily bolus-contrast MR (dynamic susceptibility contrast [DSC]) and CT methods. Techniques employing diffusible tracers include ASL perfusion MRI, H215O positron emission tomography, and stable xenon CT. This chapter will focus on ASL perfusion MRI strategies, but both DSC perfusion MRI12,13,14 and CT perfusion techniques have also been applied15 to AVM evaluation.
Arterial Spin Labeling
ASL perfusion MRI implementations fall into two basic categories: pulsed and continuous. For both, the basic principle is that arterial blood water is labeled and allowed to flow into the imaging plane(s), during which time there is T1 decay of the label. Subtraction of labeled images from unlabeled control images yields a difference image, in which measured signal change is directly proportional to CBF.16
One challenge for ASL is transit artifacts. An example of transit artifacts in ASL would be a high-grade large vessel stenosis, which results in delayed transit of label to the capillary bed so that labeled blood ultimately destined for tissue of interest never arrives or decays prior to arrival, or that label in large vessels is mistakenly included in the quantification of flow in tissue for which it is not ultimately destined.17,18,19,20,21
For ASL, the transit issues are of particular interest in the setting of AVM. The half-life of labeled arterial water is limited by T1 decay such that it usually does not survive long enough for substantial label to reach the venous side. With an AV shunt, there is rapid transit, which creates the potential for exploiting the transit artifact to directly visualize and perhaps quantify the shunt. Labeled blood in this case would no longer behave as a diffusible or microvascular tracer and would instead behave as an intravascular tracer. Assuming that brain tissue supplied by normal vasculature would see label appropriately delivered to microvasculature, such that perfusion can still be measured elsewhere, the effect is to create an angiographic map of AV shunt22,23,24 and a perfusion map of surrounding brain with ASL (Fig. 51.1).
Diagnostic Evaluation
Detection of Arteriovenous Shunt
Preliminary studies of small sample populations of patients with AVM or AVF have shown the ability to detect the presence of AV shunt at relatively high contrast. An early study including seven patients with AVM (confirmed with DSA) used a continuous ASL (CASL) approach at 3T to show conspicuously increased intensity in nidus or draining venous structures (Fig. 51.2).25 Label suggestive of shunt was not visualized in control cases of cavernoma or glioblastoma with the exception of minimal sagittal sinus label apparent near a large glioblastoma. Noguchi et al.26 used the flow-sensitive alternating inversion recovery (FAIR) technique at 3T, demonstrating visualization of prominent cortical veins over the hemisphere ipsilateral to dural AVF involving transverse sinus in seven patients, while prominent cortical veins were not visualized on the contralateral side or in patients without prominent cortical venous drainage on DSA. FAIR was more successful than routine T2-weighted images, and cortical vein findings on FAIR were concordant with DSC perfusion MRI.
A subsequent retrospective study using a pseudo-continuous ASL approach at 1.5T with background suppression and 3D fast spin echo readout included a sample population of 26 patients, 15 with dural AVF small or less than 2 cm AVM on DSA and 11 control patients with parenchymal or subarachnoid hemorrhage and DSA negative for vascular lesion.27 These authors first evaluated conventional imaging findings such as intracranial
hemorrhage, parenchymal edema, abnormal MRA, or vascular enhancement, deciding without use of ASL data whether a vascular malformation was present, and then adding ASL data and reassessing imaging data. This study showed visualization of venous arterial spin label predictive of positive DSA with sensitivity and specificity of 78% and 85%, respectively. Receiver operating characteristic analysis showed objective improvement in accuracy with the addition of ASL data (area under curve improved from 0.798 to 0.891). Of interest, two patients with DSA initially interpreted as negative showed positive findings on follow up within 1 month (one AVF and one AVM) (Fig. 51.3). Because edema and mass effect from parenchymal hemorrhage can obscure small vascular malformations, potential increased sensitivity with a technique such as ASL can provide added value to DSA (Fig. 51.4).
hemorrhage, parenchymal edema, abnormal MRA, or vascular enhancement, deciding without use of ASL data whether a vascular malformation was present, and then adding ASL data and reassessing imaging data. This study showed visualization of venous arterial spin label predictive of positive DSA with sensitivity and specificity of 78% and 85%, respectively. Receiver operating characteristic analysis showed objective improvement in accuracy with the addition of ASL data (area under curve improved from 0.798 to 0.891). Of interest, two patients with DSA initially interpreted as negative showed positive findings on follow up within 1 month (one AVF and one AVM) (Fig. 51.3). Because edema and mass effect from parenchymal hemorrhage can obscure small vascular malformations, potential increased sensitivity with a technique such as ASL can provide added value to DSA (Fig. 51.4).
False positives may occur and attention to factors that can contribute to this is important, including ASL imaging strategy and field strength. The FAIR technique, for example, uses a labeling strategy where labeled water in venous as well as arterial blood flows into the imaging volume.26,27 Also, implementations of ASL employ or have the option of employing vascular crushers to eliminate label in large vessels for accurate quantification, but crusher gradients would not be used for the purpose of shunt detection or visualization. Without suppression of label in large vessels, steno-occlusive cerebrovascular disease could lead to slow flow in arteries, which could be mistaken for venous label. Also, improvements in ASL methodology, such as higher field strengths (with longer T1 and longer label half-life), pseudo-continuous labeling, background suppression, and more (see below) can result in label surviving to normal draining veins especially in younger patients with normal or robust flow states (Fig. 51.5; Geek Boxes 1 and 2). Prominent label can also appear in benign vascular malformations, such as developmental venous anomalies, and the noninvasive ASL methodology may
prove useful in improving understanding of the vascular physiology of such anomalies (Fig. 51.6).
prove useful in improving understanding of the vascular physiology of such anomalies (Fig. 51.6).
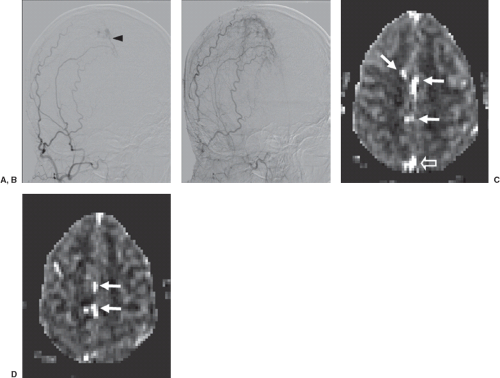 FIGURE 51.4. A 27-year-old with dural arteriovenous fistula (AVF). (A) Early and (B) delayed oblique digital subtraction angiography projections from right external carotid artery injection shows AVF at midline with primary supply from middle meningeal artery, with early venous drainage (A, black arrowhead) and more prominent venous filling on delayed view (B). (C and D) Two consecutive cerebral blood flow maps from pseudo-continuous arterial spin labeling acquisition show venous label indicating shunt in veins at midline (white arrows) and in sagittal sinus (C, open arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|