Cerebral Perfusion Studies in Substance Abuse and Dependence
Linda Chang
Santosh Yadav
Thomas Ernst
According to the World Drug Report from 2012, approximately 230 million people worldwide, or approximately 5% of the population aged 15 to 64 years, have abused illicit drugs at least once. Approximately 27 million of these drug users are considered “problem drug users,” with drug dependence or drug-use disorders. Among injection drug users, the prevalence of human immunodeficiency virus is estimated at approximately 20%, hepatitis C at 46.7% and hepatitis B at 14.6%, and approximately one of every 100 deaths among adults is attributed to illicit drug use. Therefore, illicit drug use represents a major challenge to the global disease burden. Over the past decade, coordinated efforts by many nations and the United Nations Office on Drugs and Crime have led to decreased supply and production for some of the plant-based illicit drugs (e.g., cocaine, opium), but their efforts are offset by increases in production and consumption of synthetic drugs (e.g., amphetamine-type stimulants) by illicit drug users.
Studies that used perfusion imaging to evaluate individuals who abused or are dependent on various substances have provided important insights into the neuropathophysiology of addiction and substance abuse. Some of this knowledge may ultimately lead to better treatments or preventions to reduce demands for drug abuse. This chapter focuses on how each of these types of illicit drugs or substances might affect brain perfusion, as assessed by brain imaging studies in individuals who have used these drugs. Furthermore, several legal psychoactive substances, including alcohol, tobacco, and caffeine, can influence brain perfusion when used acutely, and chronic users also demonstrate brain perfusion changes. These legal substances are often used concurrently with illicit drugs, which may further impact brain perfusion. Therefore, this chapter will also review and discuss brain perfusion abnormalities or changes associated with alcohol abuse or dependence, tobacco smoking, and caffeine use.
Imaging Techniques to Evaluate Brain Perfusion
Recent advances in brain imaging have provided high-resolution information on brain structure and function. Functional brain imaging techniques are based largely on indices of hemodynamics. Various perfusion imaging methods are used to provide quantitative estimation of blood movement that supplies oxygen, glucose, nutrients, and other necessary substrates to organs or tissues. Because brain tissue does not accumulate glucose, and brain cells can produce energy via anaerobic glycolysis only for several minutes, uninterrupted and sufficient supply of oxygen and glucose to brain tissue is needed via capillary perfusion. Many complex mechanisms and factors can alter autoregulation that maintains brain perfusion, which in turn could influence brain cell activities and function.
Hemodynamic changes can be studied using several imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET). Each of these techniques provides different sensitivity and resolution. SPECT and PET are nuclear medicine techniques that can measure perfusion or hemodynamics changes using a radiotracer, such as 15O water (for PET), 133-xenon (133Xe), 99mTc-HMPAO (99m technetium-hexamethylpropyleneamineoxime), or 99mTc-ECD (99m technetium ethyl cysteinate dimer; for SPECT); 133Xe is also used for perfusion studies with CT (see Chapter 10).
Advances in MR techniques over the past two decades have made it possible to measure brain perfusion with or without use of a nonradioactive contrast agent. Perfusion MRI techniques provide a relative or absolute measurement of the parameters of cerebral microvascularization such as cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time. Dynamic susceptibility contrast (DSC) perfusion-weighted imaging requires an exogenous contrast agent, such as gadolinium (see Chapters 7 and 8). A newer, MR-based technique, arterial spin labeling (ASL) can measure the spins in the endogenous blood and quantify the perfusion change without contrast agents. ASL can assess CBF by taking advantage of the arterial water as a freely diffusible tracer. It is completely noninvasive and repeatable and is performed without gadolinium (see Chapters 15 to 22). Brain perfusion studies of substance users employed SPECT and PET techniques in earlier studies and MR perfusion methods in more recent studies; therefore, this chapter will summarize and discuss findings from these studies.
Perfusion Studies in Opioid-Dependent Individuals
Global prevalence of opioid use in 2010 was estimated at 0.6% to 0.8% of the population aged 15 to 64 (between 26.4 and 36 million opioid users), of which nearly half
used opiates, particularly heroin (or diacetylmorphine) (Table 74.1). In North America and Oceania (Australia and New Zealand), prescription opioids are used more than heroin, whereas in Eastern Europe and southeastern Europe, opiates (heroin and, to a lesser extent, kompot, which is acetylated opium) are used more frequently. Illegal trafficking of heroin originates from Afghanistan, where much of the opium poppies are grown. However, many synthetic opioids, in particular fentanyl and buprenorphine, are abused widely as well. Opioids are “downers,” which depress the brain’s pleasure systems and interfere with the brain’s ability to perceive pain.
used opiates, particularly heroin (or diacetylmorphine) (Table 74.1). In North America and Oceania (Australia and New Zealand), prescription opioids are used more than heroin, whereas in Eastern Europe and southeastern Europe, opiates (heroin and, to a lesser extent, kompot, which is acetylated opium) are used more frequently. Illegal trafficking of heroin originates from Afghanistan, where much of the opium poppies are grown. However, many synthetic opioids, in particular fentanyl and buprenorphine, are abused widely as well. Opioids are “downers,” which depress the brain’s pleasure systems and interfere with the brain’s ability to perceive pain.
TABLE 74.1 PERFUSION STUDIES IN OPIATE OR OPIOID-DEPENDENT INDIVIDUALS | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
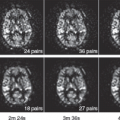
Early studies of brain perfusion in opioid users employed nuclear medicine techniques. Using 99mTc-HMPAO SPECT, chronic opiate-dependent or methadone-maintained individuals showed reduced regional CBF (rCBF) compared with nonusers in many cortical and subcortical regions, particularly the prefrontal regions.1 Furthermore, the normal hemispheric asymmetry in control subjects was reversed in the opioid users. In another SPECT study, also with 99mTc-HMPAO, perfusion defects were most prominent during early withdrawal (after 1 week), particularly in the frontal, parietal, and temporal cortices, but improved after 3 weeks of in-patient treatment in all subjects with initially abnormal scans.2 However, during a craving task with 15O-water PET, opiate-dependent individuals who had been abstinent for approximately 8 months showed increased rCBF in the left medial prefrontal and left anterior cingulate cortices, but decreased rCBF in the occipital cortex. Furthermore, the degree of craving correlated with rCBF in their left orbitofrontal cortex.3 Finally, SPECT imaging was also used to assess brain perfusion changes after intravenous infusion of heroin. 99mTc-HMPAO administered during the “rush” (15 seconds after the heroin infusion) showed increased rCBF in the left posterior cerebellar lobe, left anterior cingulate gyrus, and right precuneus, as well as during the euphoric state (tracer administered 15 minutes postheroin infusion; Fig. 74.1, left panel).4 Together, these nuclear medicine studies demonstrate that opiates are associated with alterations in brain perfusion in the acute, chronic, and abstinent states but may normalize over longer time periods.
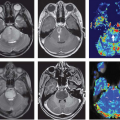
MRI with DSC, which provides higher resolution, also showed increased rCBF 80 seconds after diacetylmorphine (heroin) infusion compared to saline, but the increase was found only in the amygdala (by approximately 50%, Fig. 74.1, right panel) and not in other regions evaluated (cerebellum, thalamus, or cingulated cortex).5 Only one study used MRI with ASL to evaluate brain perfusion in methadone-maintained opiate-dependent subjects. Although no comparisons were made with nondrug users to determine whether this technique showed the abnormal rCBF that was found in prior SPECT and PET
studies, the methadone-maintained subjects who had more severe depressive symptoms had lower rCBF in bilateral frontal brain regions.6
studies, the methadone-maintained subjects who had more severe depressive symptoms had lower rCBF in bilateral frontal brain regions.6
Perfusion Imaging in Cocaine Users
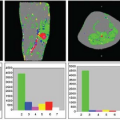
According to the 2012 World Drug Report, the global prevalence of cocaine abuse ranges from 0.3% to 0.4% (or 13.2 to 19.5 million) of the adult population aged 15 to 64 years (Table 74.2). Although the major markets for cocaine continue to be in North America, Europe, and Oceania (mainly Australia and New Zealand), cocaine is also becoming a major drug of abuse in Central and West Africa. Cocaine abuse may cause a variety of neurologic and neuropsychiatric complications, such as cerebral infarcts, intracerebral hemorrhage, ischemia, and depression.7,8,9 Cocaine is a psychostimulant that acts as a monoamine reuptake inhibitor and mediates functionality of these neurotransmitters (dopamine, serotonin, and norepinephrine) as an exogenous catecholamine transporter ligand.10 Cocaine also has significant effects on the vasculature and can cause vasoconstriction in the brain,11 leading to decreased cerebral perfusion.12,13 An earlier 133Xe inhalation study showed that 0.3 mg/kg of cocaine, but not placebo, given intravenously, produced increased CBF in the bilateral hemisphere, maximally in the frontal and parietal regions.14 However, others found that acute cocaine administration led to maximal CBF changes at 8 minutes, with a 15% to 20% decreased CBF globally and regionally in dopamine rich areas, and in a dose-dependent manner, on 15O-water PET,15 as well as approximately 30% decreased “absolute” blood flow on 99mTc-HMPAO12 and approximately 10% decreased blood flow on 99mTc-bicisate (ECD) SPECT.16 These dopamine-rich areas included prefrontal, frontal, temporal, and subcortical gray matter. Furthermore, both low-dose and high-dose cocaine led to significant decreases (>20%) in CBV and vasoconstriction of the cerebral vasculature on MRI with DSC10 and cerebral angiography.17 In addition to the direct vasoconstrictive effects of cocaine on the dopaminergic receptors on blood vessels, cocaine-induced respiratory changes resulting in reduced blood CO2 levels can also contribute to reduced relative CBV (rCBV).10 The effects of cocaine may vary regionally. A multimodal study of DSC-MRI and 133Xe-calibrated HMPAO SPECT showed that abstinent chronic cocaine abusers had increased absolute rCBF in the frontal and temporoparietal white matter (Fig. 74.2) but decreased relative rCBF in the putamen (−3.9%) and in temporal cortex (−2.4%).18 Since glial cells have a higher metabolic rate and perfusion demands than neurons, increased rCBF in the white matter of cocaine users may be a result of cocaine-mediated neuroinflammation and associated glial hypertrophy. Preclinical studies found that stimulants, such as cocaine and methamphetamine, lead to neuroinflammation through the innate immune response pathway, even at nonneurotoxic doses.19 The possibility that increased rCBF might be a result of glial activation is further supported by MR spectroscopy studies that found
higher levels of the glial marker myoinositol in the white matter of cocaine users, especially in females.20
higher levels of the glial marker myoinositol in the white matter of cocaine users, especially in females.20
TABLE 74.2 PERFUSION STUDIES IN COCAINE USERS | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Sex differences on cocaine’s effect were also found on brain perfusion. One study found no changes in CBV after cocaine administration during the follicular phase (days 3 to 8), but reduced CBV (∼10%), indicative of vasoconstriction, during the luteal phase (days 18 to 24) of the menstrual cycles of cocaine-using women.21 Furthermore, CBV was lower in men than women (with significant differences during the follicular phase) after cocaine administration.21 These sex differences may contribute to the different severity of brain dysfunction found
in chronic cocaine-abusing men and women.21 Other reports also found that cocaine-dependent women had fewer perfusion abnormalities in the frontal lobe than cocaine-dependent men, and female cocaine users had better treatment outcomes than male cocaine users.22,23
in chronic cocaine-abusing men and women.21 Other reports also found that cocaine-dependent women had fewer perfusion abnormalities in the frontal lobe than cocaine-dependent men, and female cocaine users had better treatment outcomes than male cocaine users.22,23
Lower rCBF suggestive of persistent vasoconstriction was also observed in chronic cocaine-dependent individuals. An earlier 15O-water PET study found deficits in isotope uptake (or reduced CBF) in the prefrontal cortex of chronic cocaine users, which persisted even after 10 days of abstinence from cocaine.24 Likewise, persistently decreased cerebral perfusion in abstinent cocaine users was observed on 99mTc-HMPAO SPECT,25,26 especially in the caudolateral orbitofrontal regions.27 These perfusion deficits were observed primarily in men (but not women) who were dependent on cocaine.23 With longer periods of in-patient treatment (17 to 29 days), some cocaine-dependent individuals showed improved or increased cerebral perfusion, which demonstrates that the perfusion deficits or vasoconstrictive effects of cocaine are partially reversible.25
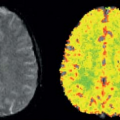
Few studies have applied ASL to evaluate cerebral perfusion associated with cocaine administration or abuse. One MRI with ASL study found a decoupling of changes in CBF and CBV during the transient state immediately following the administration of cocaine and an altered coupling of CBF and CBV during the steady state after cocaine injection.27 Another ASL study of adolescents who were exposed to cocaine prenatally found persistently reduced CBF globally and in the thalamus and occipital regions (Fig. 74.3), with possible compensatory increases in relative perfusion in the anterior and superior brain regions.28 Such long-term prenatal effects may reflect altered brain development but may also be a result of tobacco or other substance use, which is common among these adolescents.
Perfusion Studies in Users of Amphetamine-Type Stimulants
Amphetamine-type stimulants (ATS) are second only to cannabis as the most prevalent illicit drugs of abuse, with an estimated usage of 0.3% to 1.2% (14.3–52.5 million) in adults aged 15 to 64 years worldwide (Table 74.3). Demand
for ATS treatment is highest in Asia. ATS include the synthetic drugs methamphetamine, amphetamine, and ecstasy.
for ATS treatment is highest in Asia. ATS include the synthetic drugs methamphetamine, amphetamine, and ecstasy.
TABLE 74.3 CEREBRAL PERFUSION STUDIES IN AMPHETAMINE-TYPE STIMULANTS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Perfusion Studies in Methamphetamine Users
Methamphetamine (meth) is a psychostimulant that is neurotoxic in animal studies; however, its effects on perfusion in the human brain have not been studied extensively. Animal models, including rodents, baboons, and monkeys, have shown that meth can damage the dopaminergic and serotonergic nerve terminals.31 Postmortem studies of meth users also found decreased dopamine and serotonergic nerve terminal markers in the striatum.32,33 Similarly, PET studies of meth users found decreased striatal dopaminergic transporters34,35,36 and serotonergic transporters.37 Because both dopamine and serotonin can lead to alterations in vascular tone, and meth use is associated with marked increases of these monoamines in the synapses, alterations in rCBF are expected in meth users.
Two early studies found “perfusion defects” on 99mTc-HMPAO SPECT in active amphetamine or meth abusers.38,39 Such decreases in rCBF on SPECT were found even in meth users who had been abstinent for approximately 2 years.40 These findings suggest that the meth-induced vascular changes may be related to the neurotoxic effects of the drug on the nerve terminals that innervate the small cerebral vessels. MR techniques were also used to evaluate cerebral perfusion alterations in abstinent meth users41 as well as in healthy volunteers after receiving an oral dose of amphetamine.42 Compared with nondrug users, a group of abstinent meth users showed lower relative rCBF bilaterally in the subcortical gray matter (putamen/insular cortices, −10% to −12%) and the right lateral parietal brain region (−11%), but higher relative rCBF in left temporoparietal white matter (+13%), left occipital brain region (+10%), and right posterior parietal region (+24%). Male and female meth users also had opposite rCBF abnormalities in the right occipital cortex and the splenium, with increased relative rCBF in female meth users but decreased rCBF in the male users (Fig. 74.4).18 Because these meth users had been abstinent for approximately 8 months, these persistent physiologic changes in brain perfusion, along with slower reaction times on computerized tests, suggested persistent neuronal dysfunction in these brain regions. The elevated relative rCBF in the cortical regions can also be associated with the well-documented meth-induced neuroinflammation, because glial cells have higher metabolism. The gender differences in relative rCBF alterations suggested a stronger inflammatory response in female compared with male meth users.
Perfusion Imaging in Ecstasy Users
Ecstasy (3,4-methylenedioxymethamphetamine, MDMA) is an amphetamine congener that is popular as a recreational drug especially at “rave” parties. The drug is consumed orally in tablet forms; however, ecstasy often contains many impurities in addition to MDMA. Preclinical studies indicate that MDMA primarily induces release of serotonin and to a lesser degree dopamine, both of which can alter cerebrovascular tone. In addition, animal models demonstrate that MDMA induces neurotoxicity specifically to serotonergic neurons, even at low doses. Studies in human ecstasy users demonstrated altered CBF. Several studies used SPECT or PET techniques to evaluate CBF. Abstinent ecstasy users typically showed relatively normal rCBF at rest43 and rCBF changes during an attention task.44 However, naïve ecstasy users who received oral administration of MDMA (1.75 mg/kg) showed increased rCBF in the ventramedial frontal cortex, cerebellum, and superior temporal region, but decreased rCBF in the superior temporal region, insula, thalamus, and preparacentral cortex on 15O-water PET 75 minutes after taking the drug (Fig. 74.5, left panel).45 Similarly, SPECT studies of abstinent ecstasy users found persistent decreased blood flow in a dose-dependent manner in the visual cortex, the caudate, the superior parietal, and the dorsolateral frontal
regions 2 to 3 weeks after two doses of MDMA (Fig. 74.5, right panel), but those who were scanned 2 to 3 months later had higher rCBF.40
regions 2 to 3 weeks after two doses of MDMA (Fig. 74.5, right panel), but those who were scanned 2 to 3 months later had higher rCBF.40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree