Chapter 4 ARTHRITIS
IMAGING CHARACTERISTICS OF SPECIFIC DISEASES
DEGENERATIVE DISEASE
Osteoarthritis
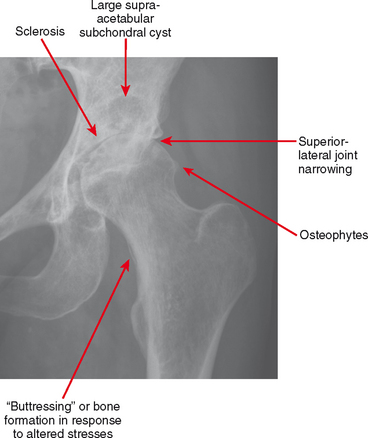
Osteoarthritis is defined by the hallmarks of osteophytes, joint narrowing, subchondral cysts and subchondral sclerosis (Fig. 4-1). However, these hallmarks are not specific for osteoarthritis.
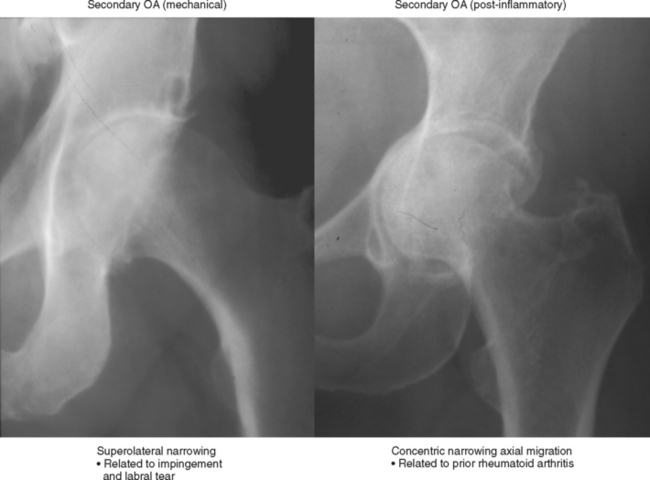
Secondary osteoarthritis is seen as a delayed consequence of some joint insult (Box 4-1). This includes diverse mechanisms such as (1) fibrocartilage or ligament injury (e.g., medial meniscal tear with medial compartment osteoarthritis; anterior cruciate ligament tear with osteoarthritis; glenoid labral tear with instability and subsequent osteoarthritis; acetabular labral tear with superolateral hip osteoarthritis); (2) articular incongruity (e.g., intra-articular fracture with malunion and articular stepoff; avascular necrosis with articular surface collapse; developmental deformity such as hip dysplasia; progressive deformity of bone as with Paget disease; malalignment such as scapholunate advanced collapse of the wrist); or (3) cartilage destruction (e.g., septic arthritis, rheumatoid arthritis or hemophilia with primary chondrolysis and subsequent osteoarthritis) (Fig. 4-2). Secondary osteoarthritis due to cartilage destruction may cause confusion because there are signs of the primary process as well as the hallmarks of osteoarthritis. A common example is chronic rheumatoid arthritis with marginal erosions and diffuse joint narrowing due to hyaline cartilage destruction and with superimposed osteophytes.
Cartilage Descriptors and MR Imaging Protocol
Detecting hyaline cartilage defects can be a challenge in certain joints even with high-field MRI. Low-field scanners are at a great disadvantage owing to low resolution, signal, and limited fat suppression. Cartilage is bright on most imaging sequences in which fat suppression is used (without fat suppression, hyaline cartilage appears intermediate to dark); the key is using a sequence with high resolution and signal-to-noise ratio (SNR) with sufficient contrast between joint fluid and cartilage. On high-field scanners the most common sequences used are protein density (PD) or T2-weighted sequences with fat suppression and two- or three-dimensional fat-suppressed T1-weighted spoiled gradient-recalled echo (GRE) sequences. The T2-weighted sequence should optimally have a PD-like echo time (TE) of around 40 to 60 msec to increase SNR while retaining high fluid conspicuity. The GRE sequence can be acquired with a flip angle of 40 to 60 degrees, using the same value for time to repetition (TR) (40–60 msec) and minimum TE, combined with fat suppression. This yields a T1-weighted sequence in which cartilage is bright and fluid is dark. Both are sensitive for cartilage loss, but the PD T2-weighted sequence is more versatile and is also useful for ligaments, tendons, and marrow. The GRE sequence is very useful in situations in which cartilage is thin or the joint is small, as in the wrist and elbow. Intra-articular contrast can be useful to demonstrate cartilage defects, but there is often insufficient difference in signal between cartilage and joint fluid containing gadolinium. Newer sequences improve detection of cartilage defects; these generally involve steady-state GRE techniques and their nomenclature is vendor-specific. Physiologic imaging techniques including T2-mapping, T1-rho imaging, spectroscopy, and delayed gadolinium-enhanced MR imaging of cartilage (dGEMRIC) can be used to map areas of dehydration and proteoglycan depletion indicative of early cartilage degeneration. These techniques are used mainly for research studies but may be incorporated into routine MR protocols in the future as treatments for early cartilage damage evolve.
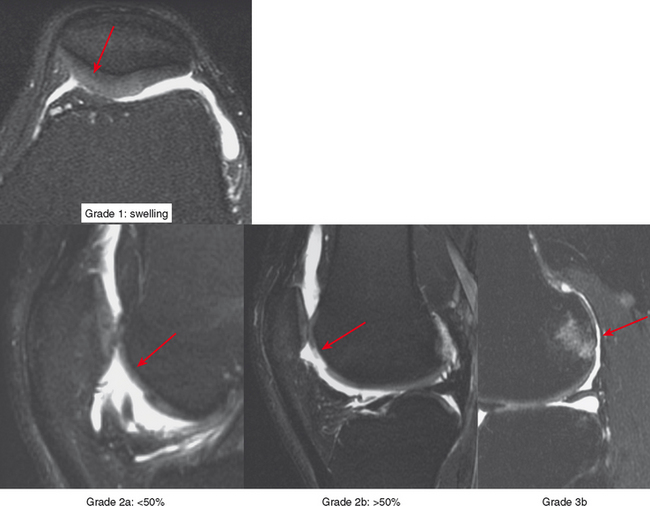
Radiologists interpreting MRI should use a common language with referring doctors and strive to be accurate and precise in describing cartilage damage. A commonly used grading system in the orthopedic nomenclature is the Outerbridge system (Box 4-2), which dates back to the late 1950s and was subsequently modified in 1975. The system was based on probing of the cartilage surface at surgery: grade 1 is cartilage softening; grade 2 is a cartilage defect smaller than half-inch wide (the width of the probe end; later changed to 1.5 cm); grade 3 is a defect greater than or equal to half-inch wide; and grade 4 is a full-thickness defect with exposed bone (Fig. 4-3). This system is awkward to apply to MRI because a combination of grades is usually present, and the surgical grading is based on surface analysis.
BOX 4-2 POPULAR GRADING SYSTEMS FOR DEGENERATIVE ARTHRITIS
Kellgren-Lawrence Radiographic Grading Scale of Osteoarthritis of the Tibiofemoral Joint
Grade 0: No radiographic findings of osteoarthritis
Outerbridge Arthroscopic Classification of Chondrosis (Modified)
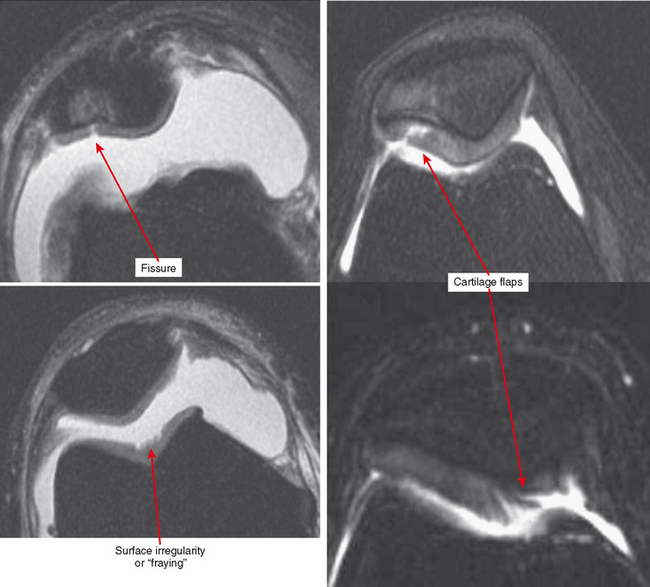
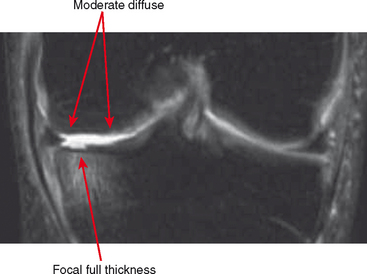
Other classification systems have subsequently been developed (see Box 4-2). The commonly used term chondromalacia is particularly useless and nondescriptive. The most useful method of characterizing cartilage loss is to be descriptive in location, size, and depth of cartilage defects seen. Cartilage loss can be divided into diffuse and focal lesions (Figs. 4-4 and 4-5). Some useful descriptors are listed in Box 4-3.
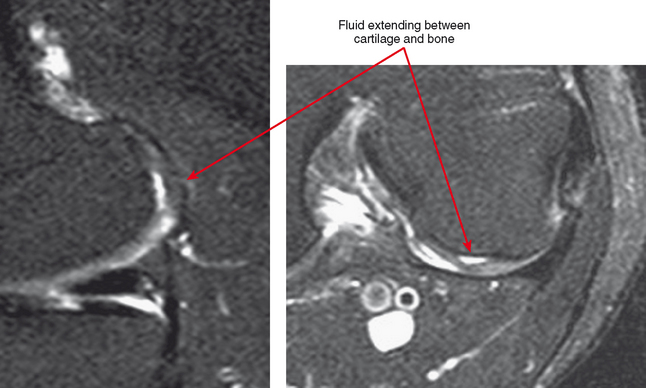
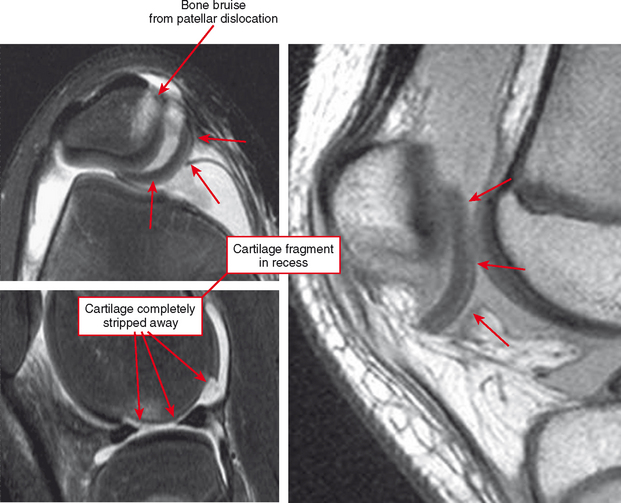
Cartilage flaps and delamination deserve special consideration. These represent unstable cartilage lesions and should be described separately. A cartilage flap is an oblique linear defect (see Fig. 4-4), causing the fragment to be potentially mobile; this can result in pain and locking, and the fragment can break off to form a body. Delamination is separation of the hyaline cartilage from underlying bone (Figs. 4-6 and 4-7). Fluid undermines the cartilage, and although the lesion may not be observed on arthroscopy, the “bubble” of fluid can propogate and form a flap.
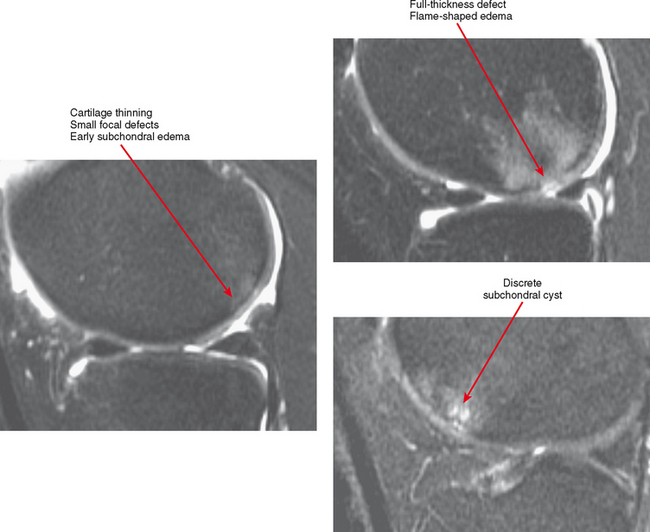
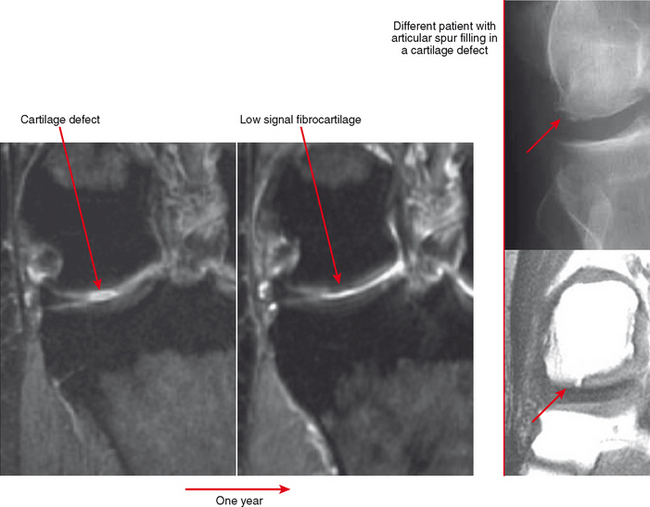
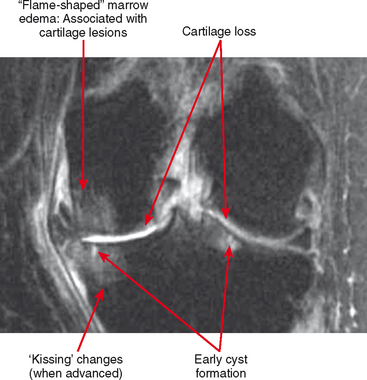
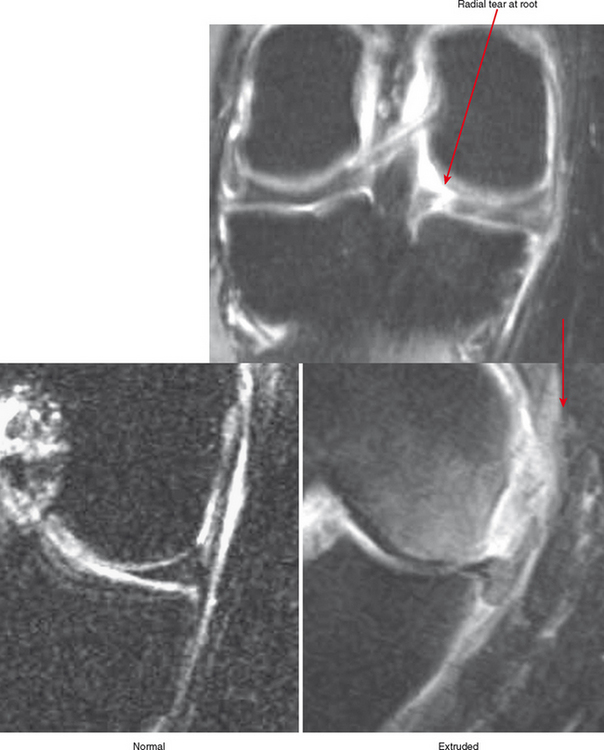
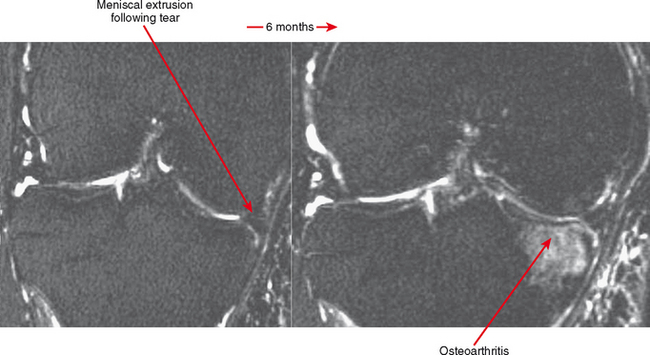
Cartilage lesions of all types can exhibit subchondral edema on MRI; this may occur only on one side of the joint in the early stages, later progressing to cyst formation (Fig. 4-8). Cartilage lesions almost always progress but occasionally spontaneously fill in with dark fibrocartilage or bone (Fig. 4-9). In the knee, a typical progression of cartilage loss is observed. A common presentation is tearing of the medial meniscus with extrusion; the altered forces cause diffuse cartilage loss in the medial compartment and osteophyte formation. Lateral compartment spurs form, with cartilage loss beginning near the tibial spines and eventually distributed throughout the compartment with a degenerative tear of the lateral meniscus following thereafter (Fig. 4-10). Meniscal extrusion and unstable meniscal tears (especially complex tears and radial tears) can result in rapid compartmental cartilage loss (Figs. 4-11 and 4-12) and should be described in the radiology report. Impingement and instability in other joints are also associated with osteoarthritis. Early osteoarthritis can also occur at the hip related to impingement and labral tear, for example.
Erosive Osteoarthritis
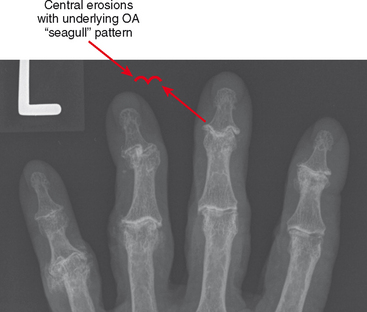
Erosive osteoarthritis, also called EOA or inflammatory osteoarthritis, is seen in patients with underlying osteoarthritis of the hands. Patients are typically elderly and often female. Arthritic interphalangeal joints (especially the distal interphalangeal joints) become tender, painful, and swollen. Central erosions occur and lead to the classic “seagull” pattern of the joint surface (Fig. 4-13). The joint may eventually undergo ankylosis.
Neuropathic Osteoarthropathy
Neuropathic osteoarthropathy, also known as Charcot arthropathy, can be considered a severe, aggressive form of osteoarthritis with a component of instability. Some features of osteoarthritis are often present, including sclerosis, osteophytes, and bodies. However, often subluxation, erosion, and joint destruction are seen, which may be extreme and deforming. The arthropathy begins as a sensory neuropathy, which may be from diabetes, syringomyelia, tabes dorsalis, leprosy, or other conditions (Box 4-4). Joints in the insensate region undergo unrecognized injury with ligament damage, osteochondral and capsular disruption and even fracture. The joint injury persists and progresses as a result of continued stress, and the arthropathy ensues. Loss of sympathetic control with neuropathy, or hypovascularity in the case of diabetic vasculopathy, may also play a role in disease progression.
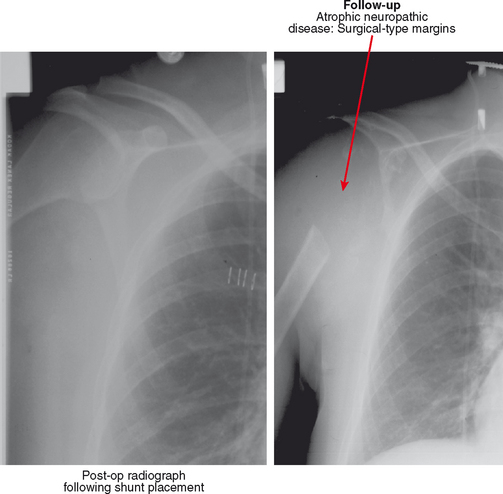
Neuropathic osteoarthropathy can be described in three forms: atrophic, hypertrophic, and mixed (Figs. 4-14 and 4-15). The atrophic form appears as well-defined osteolysis with a “surgical–like” appearance. Proliferative response is absent, but the remaining bones are normally mineralized and the margins may be sclerotic. This pattern is classically seen in the shoulders associated with a spinal cord syrinx. The hypertrophic form appears as a severe osteoarthritis with new bone production, sclerosis, fragmentation, and often subluxation. The mixed form has features of bone destruction and production.
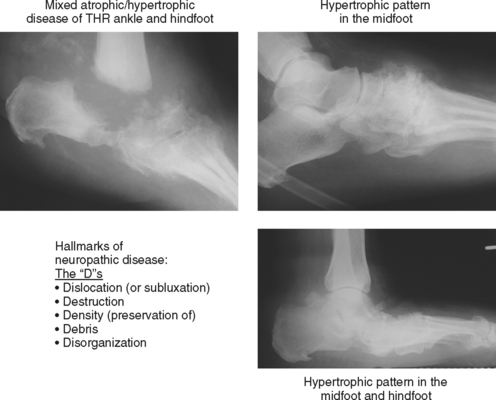
Figure 4-15 Hypertrophic and mixed forms of neuropathic disease, common in feet of diabetic patients.
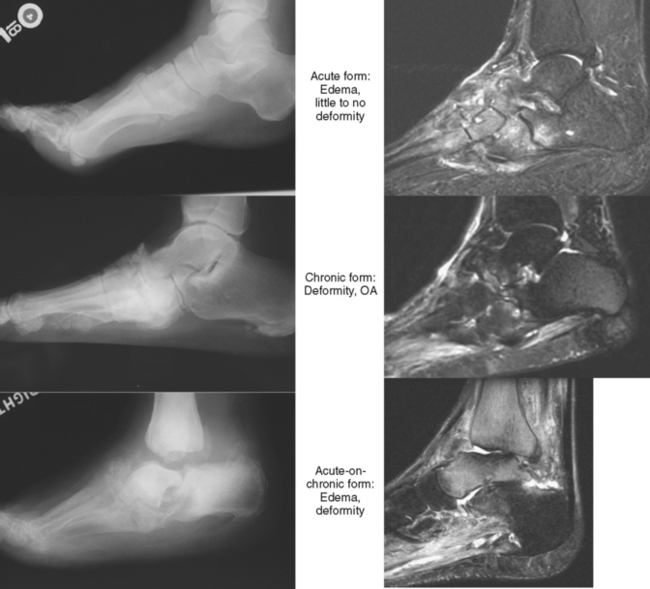
Neuropathic disease can also be described in terms of acute, chronic, or acute-on-chronic (Fig. 4-16). In acute neuropathic osteoarthropathy, the patient presents with diffuse swelling and erythema; clinically this can simulate infection. The involved joints in this early stage of disease often show little deformity or malalignment, and radiographs may show just soft tissue swelling. On MRI, joint effusions are common, with prominent subchondral edema that may extend far into the medullary cavity. Signal intensity changes in the bone marrow consisting of low signal intensity on T1-weighted images and high signal on T2-weighted images may be identical to those observed in septic arthritis and osteomyelitis. Erosions may be seen at the margins of the joint. On gadolinium-enhanced images, marrow enhancement is typically present, with predominantly subchondral distribution. Periarticular soft tissue enhancement may also be seen. Recent fractures related to neuropathic osteoarthropathy may create intense bone marrow edema, which lead to potential diagnostic pitfalls. In chronic neuropathic osteoarthropathy, radiographic features have been summarized by words beginning with “D”: destruction, debris, preserved bone density, disorganization, and dislocation (or subluxation) (Box 4-5).
The most commonly involved articulation is the Lisfranc joint. Involvement here causes the metatarsal bases to migrate dorsally, and the longitudinal arch collapses, resulting in a rocker-bottom deformity. MR imaging features are more typical of osteoarthritis, with bone production and subchondral cystic change. Acute clinical presentation may be superimposed on chronic neuropathic disease, resulting in features of both on radiographs and MRI. In any phase of neuropathic disease, in the setting of diabetes there is often underlying diffuse soft tissue edema or diffuse muscle atrophy, which in the absence of contrast can appear similar to diffuse cellulitis. Marrow changes can simulate infection.
In addition, osteomyelitis is frequently associated with neuropathic disease of the foot. Chronic neuropathic deformity causes osseous protuberances that can produce increased friction with ill-fitting footwear and results in adjacent skin callus formation, breakdown, and ulceration; osteomyelitis ensues from contiguous spread. Typical locations for this to occur are the metatarsal heads, midfoot, and calcaneus. Differential features between neuropathic disease and infection are discussed in detail in Chapter 5.
INFLAMMATORY DISORDERS
Septic Arthritis
Infection should always be considered when a monarticular arthropathy is seen. Imaging can appear identical to other inflammatory arthropathies such as rheumatoid arthritis or gout. Specific findings are discussed in more detail in Chapter 5.
Rheumatoid Arthritis
Rheumatoid arthritis (Figs. 4-17 through 4-22) affects females more often than males at a ratio of 2–3 to 1. Typically, adult-onset rheumatoid arthritis occurs in the 20s to 40s. Symptoms at presentation classically include joint stiffness, especially in the morning, with pain and swelling—most commonly polyarticular and symmetric. Systemic manifestations include fatigue and weight loss. Clinical criteria for diagnosis include at least four of the following (criteria 1 through 4 must have been present for at least 6 weeks): (1) morning joint stiffness lasting at least 1 hour; (2) soft tissue swelling of at least three joints; (3) swelling of the proximal interphalangeal, metacarpophalangeal, or wrist joints; (4) symmetric involvement; (5) subcutaneous nodules; (6) positive rheumatoid factor; (7) radiographic evidence of erosions of the joints of the hand or wrist. Most patients (approximately 85%) have positive serum rheumatoid factor.
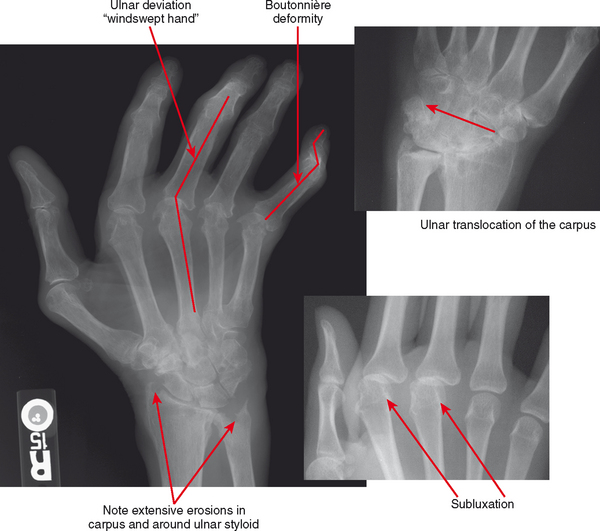
Figure 4-18 Hand deformities in rheumatoid arthritis. Boutonnière deformity is flexion at the proximal interphalangeal (PIP) joint and extension at the distal interphalangeal (DIP) joint; swan-neck deformity is extension at the PIP and flexion at the DIP. Deformities are due to stretching or erosion of joint cartilage and capsular structures and supporting ligaments with imbalance of forces across the regions. Also not pictured are dorsal intercalated segmental instability and volar intercalated segmental instability deformity and other carpal malalignments that occur with intercarpal and capsular ligament insufficiency (see Chapter 9). Ulnar translocation occurs when the extrinsic ligaments erode, allowing the carpus to shift over the ulna.
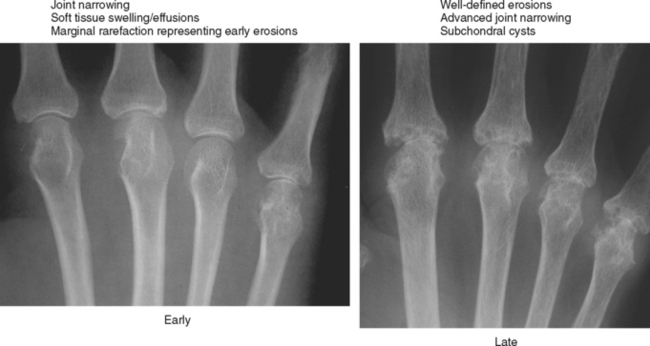
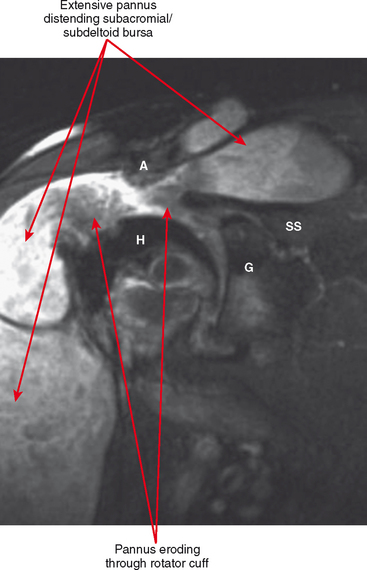
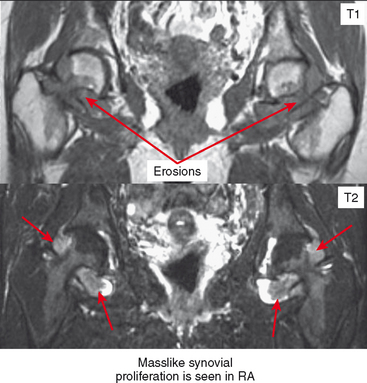
Periarticular osteopenia is a classic radiographic feature, especially in early stages of rheumatoid arthritis of the hands and feet, although a generalized pattern of osteopenia can also occur. Involved joints generally demonstrate concentric or uniform joint narrowing related to diffuse cartilage loss. However, in weight-bearing joints there may be more severe narrowing at the weight-bearing surface. Axial migration can occur at the hips because of bone remodeling at the central portion of the acetabulum, with inward bowing of the iliopectineal line, called protrusio acetabuli. Characteristic marginal erosions result from thickened, inflammatory synovial tissue (pannus) eroding the bone at the bare area adjacent to the margin of the articular cartilage. Osseous proliferation is not a feature of rheumatoid arthritis; however, osteophyte formation can occur in longstanding rheumatoid arthritis as a result of superimposed secondary osteoarthritis.
Deformities of the hands and feet are common in rheumatoid arthritis for a variety of reasons: laxity and distention of the joint capsule, ligamentous laxity or disruption, tendinopathy or tendon tears, and altered muscle tone. The “swan neck” deformity is hyperextension at the proximal interphalangeal joint and flexion at the distal interphalangeal joint. The “boutonière” deformity is flexion at the proximal interphalangeal joint and hyperextension at the distal interphalangeal joint. These deformities result from imbalance of the flexor and extensor tendons. Subluxations at the metacarpophalangeal and metatarsophalangeal joints are also common: The digits of the hands deviate in an ulnar direction (“windswept hand” appearance); in the foot, hallux valgus is very common and may be severe, leading to overlap of the first and second toes. In the hands, the carpal bones commonly erode (carpal-dominant involvement), with ligamentous disruption and laxity causing carpal instability patterns. In more severe cases, the process of erosion and instability may reach a point at which there is “carpal collapse,” with the metacarpal bases nearly apposed to the radius. The entire carpus and hand may slip in an ulnar direction, referred to as “ulnar translocation.” Carpal collapse and dissociation, in addition to mass effect from pannus, can cause impingement on the median nerve as it passes through the carpal tunnel, resulting in carpal tunnel syndrome.
Spine Involvement
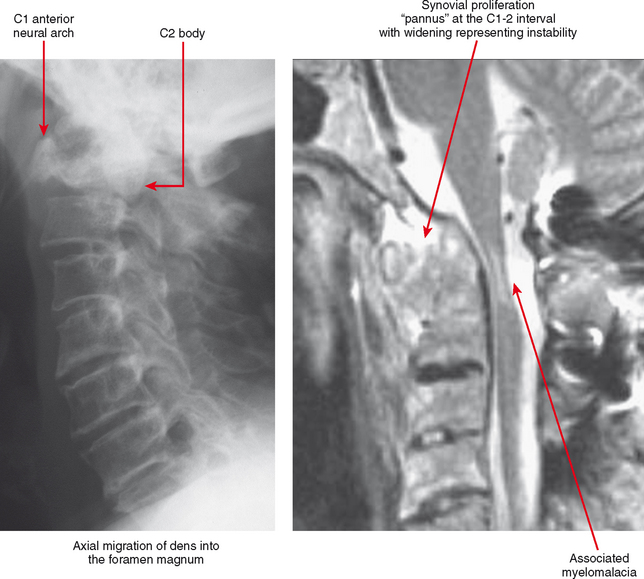
A major site of serious musculoskeletal complications from rheumatoid arthritis is the cervical spine. The dens is surrounded by synovial tissue, anteriorly at the junction of the dens with the anterior arch of C1, and posteriorly at the transverse ligament. Pannus at these synovial locations and at the facet joints can cause laxity of the transverse ligament and erosion of the dens itself, leading to excessive motion and instability at C1-C2. The instability may not be apparent on a neutral lateral view; lateral views in flexion and extension are generally indicated to gauge the degree of instability, although the flexion/extension motion must be performed with great caution, always allowing patients to flex and extend the neck voluntarily, determining their own limitation. This instability is very important to identify in individuals at risk, because relatively minor trauma in this setting can cause a high cervical cord injury. This is especially a concern in the preoperative patient, since before and during surgery the neck is extended and flexed during intubation and anesthesia.
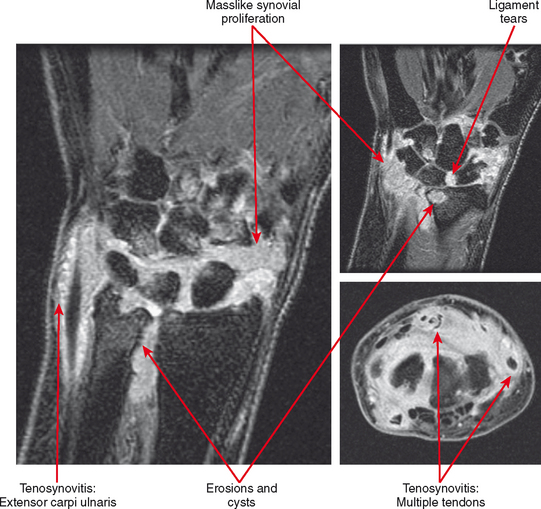
Tenosynovitis and bursitis are common in persons with rheumatoid arthritis. Often multiple tendon sheaths are involved around the joint. If synovitis of a joint and of multiple adjacent tendon sheaths is observed, a diagnosis of rheumatoid arthritis should be entertained. In rheumatoid arthritis, synovial pannus can be observed within the tendon sheaths, with complex fluid seen on T2-weighted images, with marked distention of the sheath. Similarly, distention of periarticular bursae, such as the olecranon bursa and retrocalcaneal bursa may occur, seen on MRI as complex fluid signal representing pannus within the bursal capsule. In chronic rheumatoid arthritis, intra-articular bodies are occasionally observed and may be seen as multiple fibrous ovoid structures within the joint or tendon sheath known as “rice bodies” because of their shape, quantity, and whitish appearance at surgery. Rheumatoid nodules may be observed. On MRI rheumatoid nodules are well-defined, located in the subcutaneous fat, with low signal on T1-weighted images and variable T2 signal. Diffuse enhancement may also be seen. In the spine, MRI is useful for determining the extent of pannus formation at C1-C2, as well as the degree of compromise of the spinal canal.
Juvenile Chronic Arthritis (Fig. 4-23)
Patients with Still disease can present with a variety of forms: systemic (10%), polyarticular (40%), and pauci- or monarticular (50%). Onset of the systemic form is acute and often occurs in children younger than 5 years old. Systemic manifestations of the disease include fever, anorexia, rash, lymphadenopathy, hepatosplenomegaly, pericarditis/myocarditis, arthralgias and myalgias, and anemia/leukocytosis with increased sedimentation rate. Symmetric articular involvement is typical in the polyarticular form, with wrist joints, metacarpophalangeal and proximal interphalangeal joints of the hands, knee and ankle joints, intertarsal, metatarsophalangeal and interphalangeal joints of the feet, and the cervical spine articulations commonly involved. A monarticular or pauciarticular (four or fewer joints) pattern is often seen initially, which may progress to the polyarticular form. There are early-onset and late-onset subtypes; early-onset may occur in a child as young as 1 to 5 years of age. Joints commonly involved include the knee, ankle, elbow, wrist, and cervical spine. Systemic involvement is infrequent.
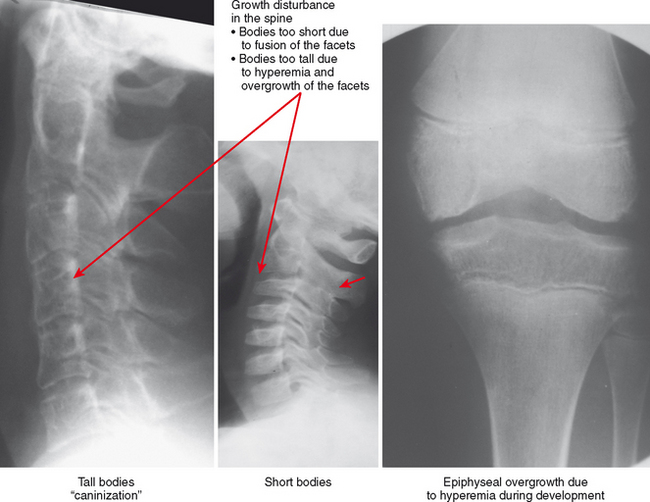
Hyperemia leads to overgrowth and an enlarged appearance of the epiphyses compared with the metaphyses and diaphyses (hemophilia can result in the same pattern). The hyperemic effect can also result in early fusion of the growth plate, causing an abrupt transition between the epiphysis and diaphysis. Early physeal fusion can cause growth disturbance, with bone shortening if the plate fuses uniformly or with deformity if the fusion affects only part of the growth plate. The diaphyses of affected extremities may be gracile, and the combination of thin diaphyses and epiphyseal overgrowth with abrupt transition results in an overtubulated pattern.