Fig. 5.1
(a, b) Abdominal ascites
How to Perform a FAST
Position
Placing patients in the Trendelenburg position increases the sensitivity of FAST to assess the presence of intra- abdominal fluid.
Ultrasound Probe
A probe of a low frequency (1–5 MHz) is used for better penetration of tissues in the abdominal cavity. Either a curvilinear or a phased array probe can be used for this purpose
Evaluation of the Pericardium and the Vena Cava: Subxyphoid Window
Place the probe in the subxiphoid space probe marker to the right, using the liver as an acoustic window.
Adjust the depth to allow viewing of the rear of the pericardium.
This view allows for visualization of the four cardiac chambers and the vena cava (Video 5.1).
Evaluation of Hepatorenal Space
Place the probe in the anterior axillary line at the bottom of the rib cage with the result of the probe head pointing in a coronal plane.
Move the probe cranially and flow in this or the mid-axillary line until the interface between the liver and kidney is clear.
Intraperitoneal fluid appears as a hypoechoic or anechoic band (black) in the hepatorenal space (Video 5.2).
Evaluation of Splenorenal Space
Place the probe in the middle or posterior axillary line at the bottom of the rib cage, with the result of the probe facing the head in the coronal plane.
Note that the left kidney is anatomically positioned higher than the right kidney; therefore, the probe is placed in more cephalic position to see the interface.
Intraperitoneal fluid appears as a hypoechoic band in black splenorenal interspace, or on top of the spleen in some instances (Video 5.3).
Bladder View
This space should be evaluated in both the longitudinal and transverse plane. Ideally, the bladder is filled to serve as an acoustic window in the space behind the bladder.
Place the probe above the pubic bone with the probe mark pointing to the right side of the patient and assessing free fluid (it will look like a black line).
Rotate the probe 90° to the right so that the points of the probe marker toward the head of the assessment in the longitudinal plane (Video 5.4).
Abdominal Paracentesis
Abdominal paracentesis in the surgical intensive care unit patient can be both diagnostic and therapeutic. A simple aspiration will often aid in diagnosis as it allows for examination of the quality and character of the fluid. Ultrasound guided paracentesis can be performed in the majority of patients as overall risks are low, and there are no absolute contraindications to this procedure [2]. Risks associated with the procedure are rare but do include: damage to intra-abdominal organs, and rectus sheath hematomas [7]. The placement of nasogastric tubes and Foley catheters aid in the prevention of damage to these organs, and blood products should be administered to patients with moderate to severe coagulopathies to reduce rectus sheath hematoma formation [2]. To estimate the amount of fluid present in the abdomen, measure the amount of fluid visible around the intestine. In general, for every 1 cm of fluid visualized approximately 1 L of fluid is present [2] (Fig. 5.2).
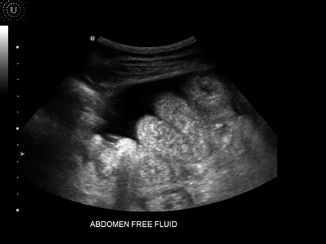

Fig. 5.2
Abdomen free fluid
To perform an abdominal paracentesis , the patient is first positioned supine and in reverse Trendelenburg to aid in the concentration of the free fluid into the pelvis. A standard abdominal curvilineal 3.5- to 5-MHz transducer is used to then identify the intra-abdominal fluid and visualize any surrounding structures. Typically the bilateral lower quadrants, lateral to the rectus sheath, are the location of choice for this procedure. This avoids the inferior epigastric artery and allows for fluid removal from the more dependent part of the abdomen. In addition, it is important in patients with parenchymal liver disease to be watchful for superficial collateral vessels or varices [7]. The right and left sides are both assessed for the largest amount of fluid present without encroaching bowel. After the site is chosen, the patient is prepped and draped in a sterile fashion, including the ultrasound transducer and local anesthesia obtained. Needle size is often determined by the purpose of the procedure. A smaller needle such as a 22 gauge is adequate when a diagnostic paracentesis is to be performed, as volumes as low as 200 ml are sufficient for laboratory examination [2]. However, if the purpose of the paracentesis is to drain a large quantity of fluid, a larger needle such as an 18 gauge may be more appropriate as it allows faster egress of the ascites. Once the appropriate needle size is chosen, a “Z-tract method” is often recommended for its insertion. This method is described as applying tension to the skin in a caudad fashion during the insertion of the needle, then once the epidermis and dermis are penetrated releasing this pull on the tissue while the needle advances through the muscle and into the peritoneum [2]. The purpose of this method is to prevent leakage of ascites after the paracentesis. Negative pressure is applied to the syringe during the entire advancement of the needle into the peritoneum. In addition, this advancement is visualized with the ultrasound, ensuring that the needle does not get advanced into an intestinal loop. Once the needle is safely in the peritoneal cavity, fluid is either aspirated for diagnosis or drained for therapy. In order to safely drain large amounts of fluid, it is recommended that a catheter be placed into the peritoneum utilizing the Seldinger technique [8].
Patients in the surgical intensive unit can develop intra-abdominal abscesses for a variety of reasons including abdominal trauma and missed injuries as well as surgical complications such as enteric leaks [9]. Although there are limitations, ultrasonography is an important tool in the diagnosis and treatment of intra-abdominal abscesses in critically ill patients. Some of the limitations for this procedure are patients who are obese, have an uncorrectable coagulopathy, extensive abdominal wounds, or an abscess located deep within the abdomen. However, when the abscess is superficial, non loculated and easily accessed without potential damage to a surrounding structure, ultrasound guided abscess drainage is the ideal method (Fig. 5.3).
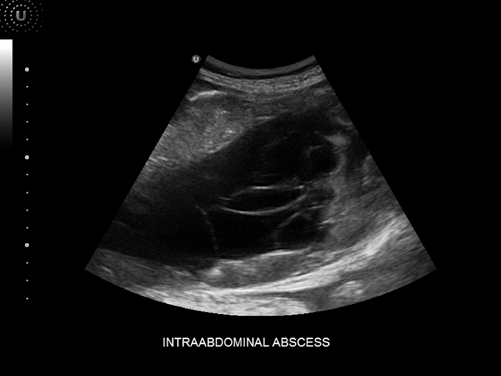

Fig. 5.3
Intraabdominal abscess
After pre-procedural localization of the intra-abdominal fluid collection has been performed utilizing the standard abdominal curvilinear 3.5-MHz or 5-MHz probe, the choice of transducer for the procedure is made [7]. A higher frequency probe (7.5–10 MHz) is used for more superficial collections while a lower frequency probe (3.5–5 MHz) is used for deeper collections [9]. The skin is then prepped and draped in sterile fashion, again including the ultrasound transducer. Due to the viscous nature of the fluid likely encountered, a larger needle such as an 18 gauge, is used for this procedure after local anesthesia has been obtained. The needle is advanced into the peritoneal cavity avoiding the epigastric arteries within the abdominal wall and under real time visualization with the ultrasound. Negative pressure to the attached syringe is applied once the needle enters the dermis, and once fluid is encountered, a guidewire placed through the needle. The needle is then removed leaving the guidewire in place inside the abscess , and a size 6- to 12-Fr catheter is then placed over the guidewire into the collection [9]. The catheter is then secured to the skin typically using suture, and a collection bag attached. The fluid can then be sent for culture and laboratory examination.
Evaluation of the Gallbladder
Acute right upper quadrant pain is a common complaint bringing patients to the emergency department. However, gallbladder pathology can also develop in patients hospitalized for completely unrelated conditions, and can result in significant morbidity and mortality in already critically ill patients in intensive care units.
Cholelithiasis is a common disease that affects from 10 to 20 % of the population during their lifetime [10]. Obesity, female gender, increasing age and genetics all play a role in the development of cholelithiasis. Although only 1 to 4 % of patients with cholelithiasis become symptomatic annually, complications include pancreatitis, biliary obstruction, acute cholecystitis and cholangitis [10, 11].
On ultrasound , gallstones can have a varied appearance dependent upon the composition of the stones. Regardless of composition however, all stones on ultrasound must move with a change in patient position and produce a shadow [12] (Fig. 5.4).
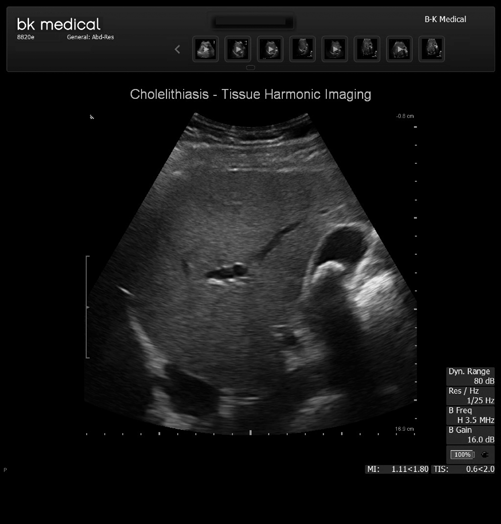

Fig. 5.4
Cholelithiasis
Choledocholithiasis occurs in approximately 8 to 10 % of patients with cholelithasis and is a significant complication [13]. This occurs when a stone migrates from within the gallbladder into the common bile duct. Although ultrasound may not always be able to detect actual stones in the common bile duct, it is useful in detecting biliary obstruction. When the common bile duct is dilated, or greater than 1 cm in diameter, choledocholithiasis should be suspected. In fact, as the common bile duct dilates and it is visualized next to the portal vein, a double channel or parallel channel sign often results [12]. In order to ensure that it is indeed a dilated common bile duct and not the hepatic artery, color Doppler can be used. As biliary obstruction progresses, the biliary tree within the liver parenchyma also dilates, and can be appreciated on ultrasound. At times the shape of the obstructed end can signify the etiology. A tapered end is more consistent with a stone as a source of the obstruction in comparison to a blunt, abrupt end which is more consistent with a tumor, likely in the head of the pancreas [12] (Figs. 5.5 and 5.6).
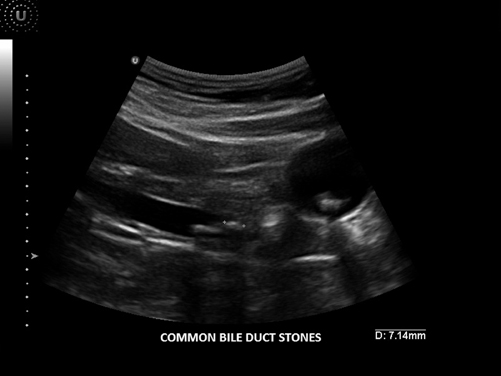
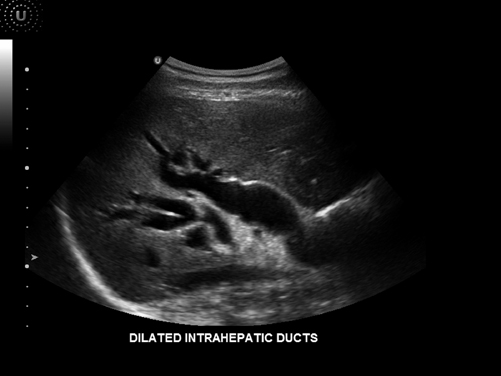

Fig. 5.5
Common bile duct stone

Fig. 5.6
Dilated intrahepatic ducts
Acute cholecystitis is known to be fairly common, and has a prevalence of 5 % in patients presenting to the emergency department with abdominal pain [14]. However, acute cholecystitis is also a well-recognized entity in critically ill patients in the intensive care unit. Although the pathology may be similar, the presentation, physical examination, diagnosis and treatment may alter significantly in the intensive care unit setting. The majority of cases in the outpatient setting are caused by stones as compared to only about 10 % of cases in the intensive care unit [15] . In contrast, acalculous cholecystitis is uncommon in the outpatient setting, accounting for only 5 to 15 % of cases, while the majority of cases in the intensive care unit are unrelated to the presence of gallbladder stones [15].
In 1844, acalculous cholecystitis was first reported in a patient having died secondary to gallbladder perforation after a femoral hernia repair [16]. The overall incidence of acalculous cholecystitis has been estimated between 0.2 to 10 % of critically ill patients and although the etiology unknown, is associated with prolonged fasting, use of total parenteral nutrition, trauma, major surgery, extensive burns, sepsis, multiple transfusions and shock [9, 15, 17]. Clinical diagnosis of acalculous cholecystitis is particularly difficult and often unrecognized in intensive care units because these patients are often mechanically ventilated, under sedation, or have undergone major surgery. In addition, traditional symptoms such as right upper quadrant pain and fever may be altogether absent [15]. In fact, it is estimated that 40 to 100 % of cases of acalculous cholecystitis are advanced, with gangrene, empyema or perforation at the time of diagnosis [15].
Ultrasound plays a critical role in the diagnosis of this condition as it is noninvasive, timely, and portable without ionizing radiation, all factors which are important in critically ill patients. In addition, the use of ultrasound allows for evaluation of surrounding structures such as the liver and kidney. The overall sensitivity and specificity reported for ultrasound in the diagnosis of acute cholecystitis both range from 80 to 88 % [18, 19].
Ultrasound findings common in this condition include a Murphy’s sign, distended gallbladder , gallbladder sludge, pericholecystic fluid, and a thickened gallbladder wall [20]. Laing et al. first described an sonographic Murphy’s sign as maximal tenderness when the sonographer presses the ultrasound directly against the visualized gallbladder in 1981 [21]. The sonographic Murphy sign alone however has a relatively love specificity and may altogether be absent, especially in acalculous cholecystitis [22




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree