Fig. 32.1
Suprapubic ultrasound evaluation of the prostate in an elderly man with advanced stage BPH. Axial (a) and sagittal (b) views show an enlarged rounded prostate bulging into the bladder base with intravesical protrusion (*)
Ultrasound measurement of bladder wall thickness has been proposed as a useful diagnostic parameter in patients with bladder outlet obstruction and other voiding dysfunctions (Kojima et al. 1997a, b). According to Franco, suprapubic ultrasound evaluation of detrusor wall thickness and intravesical prostatic protrusion is a simple, noninvasive, accurate means to assess bladder prostatic obstruction in patients with LUTS due to BPH (Franco et al. 2010). Data from other investigators, however, show that bladder wall thickness cannot reliably predict bladder outlet obstruction or detrusor overactivity, and therefore, these measurements do not provide an alternative to urodynamic studies (Blatt et al. 2008). Since reliable data on inter- and intra-observer variability, as well as reproducibility, are still lacking, measurement of bladder wall thickness is not currently part of the recommended diagnostic work-up of patients with LUTS.
A large body of evidence documents the accuracy of TRUS in calculating the volume of the prostate (Aarnink et al. 1996, 1998). However, prostate size has shown only a weak correlation with bladder outlet obstruction, while the anatomic configuration of the prostate has been found to correlate with bladder outlet obstruction and acute urinary retention. The most important parameter is the intravesical prostatic protrusion (Fig. 32.2) (Chia et al. 2003; Tan and Foo 2003; Keqin et al. 2007; Mariappan et al. 2007). Moreover, the normal triangular-shaped appearance of the prostate is changed by the continuous growth of the transition zone (Watanabe 1998; Doo and Uh 2009). A transverse section of the normal prostate shows a thin triangular shape, while that of BPH gives a semilunar shape in its early stage and a round shape in its advanced stage (Fig. 32.3). Several parameters have been introduced to quantify these changes. In particular, the degree of progression of the pathological state in BPH can be estimated by calculating how closely the shape of the prostate approaches a circle. According to Watanabe, pathological residual urine is seen if the presumed circle area ratio (i.e., the ratio of the area of the maximum horizontal section of the prostate to the area of the encompassing circle) is greater than 0.75 (Watanabe 1998). The transition zone index, i.e., the ratio between the volume of the transition zone and the total prostate volume, is also correlated with urodynamic parameters (Kaplan et al. 1995).
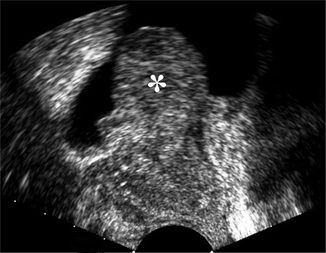
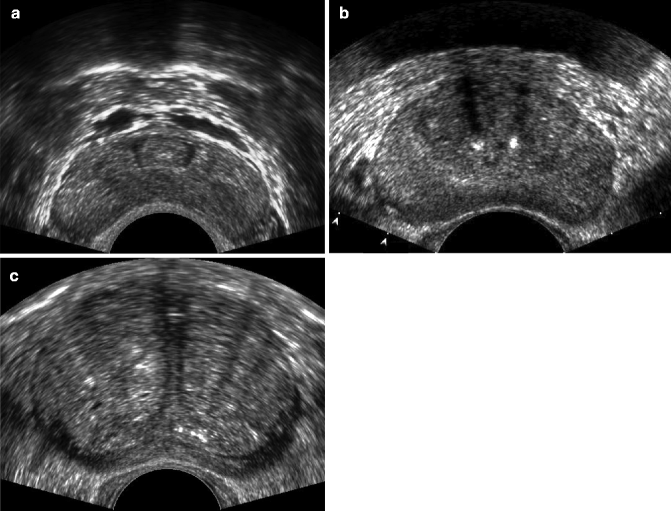

Fig. 32.2
Anatomical configuration of the prostate in an elderly man with BPH at an advanced stage and bladder outlet obstruction. Sagittal TRUS scan shows a large intravesical protrusion of the prostate gland (*)

Fig. 32.3
Anatomical configuration of the normal prostate in a young man (a) and in elderly men with early stage BPH (b) and advanced stage (c). Axial TRUS scans. The normal shape of the prostate becomes progressively semilunar (b) and then rounded (c)
32.2.1.2 Magnetic Resonance Imaging
BPH has a characteristic appearance on MRI. The signal intensity of the central gland varies considerably, depending on the ratio of glandular to stromal tissue. T2-weighted images show multiple high-signal-intensity nodules, representing hyperplastic glands with retained secretions. The nodules are surrounded by a low-signal-intensity pseudocapsule. In extensive BPH, there may be marked compression of the peripheral zone, leaving only a thin peripheral band of high signal intensity (Fig. 32.4).
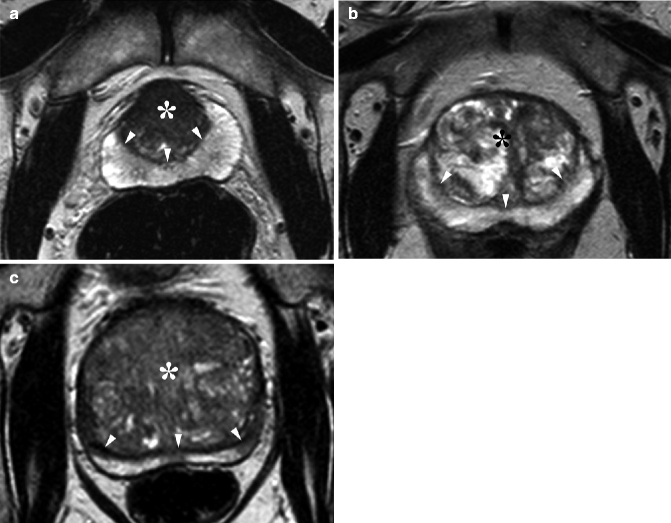

Fig. 32.4
Anatomical configuration of the prostate in a 45-year-old man with early stage BPH (a) and in elderly men with BPH in progressively advanced stages (b, c). Axial T2-weighted MRI shows progressive enlargement of the central gland (*). The gland displays variable signal intensity, depending on prevalence of fibrous and muscular tissue (a) and the hyperplastic glands with cystic components and retained secretions (b, c). The central gland is separated from the high-signal-intensity peripheral zone by a low-signal-intensity pseudocapsule (arrowheads). In extensive BPH, the peripheral zone is progressively compressed (b, c)
While the application of MRI in patients with prostate cancer has been well investigated, only a very limited number of studies have explored its use in normal prostate and in patients with BPH and LUTS.
One study evaluated T2, T1, and microvascular characteristics with dynamic contrast-enhanced MRI in patients with BPH (Kershaw et al. 2009). Results were that measurement of T1, T2, and perfusion parameters was reproducible and significantly different in the central gland and in the peripheral zone of the prostate. Other studies (Mulkern et al. 2006; Riches et al. 2009; Shinmoto et al. 2009) investigated normal prostate, BPH, and prostate cancer using diffusion-weighted imaging acquired over an extended b-factor range and processed using a monoexponential and a biexponential approach. They found a significant difference between the diffusion parameters measured with the two approaches, showing that tissue perfusion significantly influences diffusion-weighted imaging signal decay in prostate gland.
32.2.2 Evaluation of the Efficacy of Medical Treatment
The current standard of care in men with moderate to severe BPH and LUTS is treatment with an alpha-1-adrenergic blocker; in men with an enlarged prostate, a 5-alpha-reductase inhibitor is used in monotherapy or in combination. Although they are effective, each of these drug classes can produce unwanted side effects, including dizziness, asthenia, ejaculatory problems and nasal congestion, orthostatic hypotension, and sexual dysfunction. Side effects may be exacerbated by combination therapy (McConnell et al. 2003). Several clinical placebo-controlled trials have investigated the potential of phosphodiesterase type 5 (PDE5) inhibitors, which are licensed worldwide for the treatment of erectile dysfunction, to improve symptoms of men with BPH and LUTS with limited side effects. In particular, 5 mg tadalafil once daily demonstrated improvement in symptoms of LUTS and health-related quality of life compared to that of placebo (Donatucci et al. 2011). The US Food and Drug Administration recently approved tadalafil to treat the signs and symptoms of BPH and for the treatment of BPH and erectile dysfunction when the conditions occur simultaneously. Approval for prostatic use is expected in the near future in Europe as well.
Criticism of the use of PDE5 inhibitors in LUTS has centered on the lack of precise understanding of the mechanism of action (Kaplan and Hatzichristou 2007). Preliminary investigations used Doppler techniques and contrast-enhanced ultrasound to gain some insight into the vascularity of pelvic organs and to investigate the mechanism of actions of PDE5 inhibitors in patients with BPH and LUTS (Leventis et al. 2001; Berger et al. 2005; Pinggera et al. 2008a, b). Modalities used for this purpose included measurement of arterial resistive index from spectral Doppler blood flow waveforms, quantitative analysis of color pixel intensity from color Doppler imaging, and analysis of time-intensity curves after microbubble ultrasound contrast administration (Bertolotto et al. 2009). They have shown reduced blood flow and perfusion within the prostate and bladder neck in men with BPH compared with healthy controls (Kojima et al. 1997a, b; Berger et al. 2005; Pinggera et al. 2008a, b). Both surgical and pharmacological treatment with alpha-blockers improved perfusion (Kojima et al. 1997a, b; Tsuru et al. 2005; Pinggera et al. 2008a, b). Acute (90 min) treatment with tadalafil 20 mg resulted in a statistically significant increase in prostatic perfusion compared with pretreatment values (Bertolotto et al. 2009).
Due to intrinsic multiparametric capabilities, MRI has the potential to noninvasively investigate perfusion and tissue changes occurring in the prostate gland and adjacent organs as an effect of medical treatment. In particular, changes in water content and in tissue oxygenation can be estimated, as well as perfusion changes and those affecting the microscopic movement of water, such as variations in the composition and viscosity of the prostatic fluids. Dynamic contrast-enhanced MRI has been used in an animal model of BPH to assess response to drug treatment (Jia et al. 2006); no studies have evaluated the effect of medical therapy in men.
32.2.3 Arterial Embolization
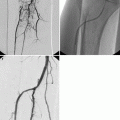
Recently innovative technologies have been introduced to improve outcomes and minimize patient discomfort and morbidity when managing BPH. Among them, prostatic arterial embolization has been suggested. Animal studies in pigs and dogs (Sun et al. 2008; Jeon et al. 2009) and preliminary investigations in men (Pisco et al. 2011) have shown that this procedure is safe, with no related sexual dysfunction, and can induce prostatic volume reduction. Detailed knowledge of prostatic arterial anatomy is necessary when performing embolization for BPH to allow identification and selective catheterization of prostate arteries, while minimizing the risks of untargeted embolization such as ischemic complications to the bladder or other pelvic organs. Multidetector CT can be used to obtain an accurate vascular map of the prostate in these patients (Bilhim et al. 2011). During follow-up, MRI can accurately investigate changes in prostate volume, as well as vascular changes and possible procedure-related complications (Fig. 32.5).
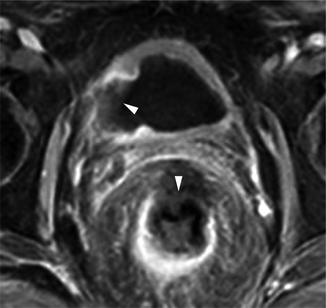

Fig. 32.5
Prostatic arterial embolization to treat BPH complicated with bladder and rectal ischemia. Image from dynamic contrast-enhanced acquisition obtained after the embolization procedure shows a non-enhancing zone in the right lateral bladder wall and in the anterior portion of the rectal wall (arrowheads)
32.2.4 Minimally Invasive Procedures
In the last decade, minimally invasive procedures have become the most popular options for surgical treatment of BPH with LUTS (Berardinelli et al. 2009). They include, but are not limited to, transurethral laser procedures, transurethral microwave therapy, transurethral needle ablation, transurethral ethanol ablation of the prostate, and high-intensity frequency ultrasound. TRUS and MRI allow assessment of prostate volume and shape and may have a role in patient selection. Several preclinical and a few preliminary clinical applications show that the avascular volume created by ablative therapy manifests as an area where tissue perfusion is absent (Huang et al. 2006; Liu et al. 2006; Cheng et al. 2008; Atri et al. 2009). Blood flow monitoring after focal ablative therapy with MRI imaging or contrast-enhanced ultrasound is therefore potentially beneficial because it allows identification of the ablated regions of the parenchyma (Fig. 32.6).
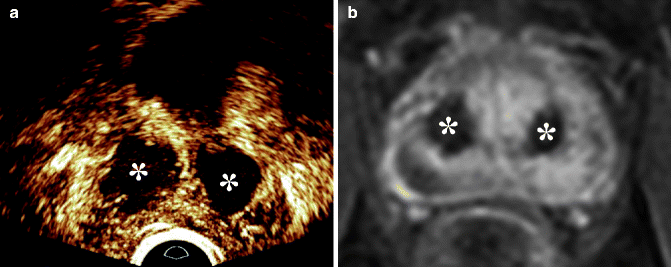

Fig. 32.6
Laser ablation of the adenoma to treat BPH. (a) Endorectal contrast-enhanced ultrasound and (b) dynamic contrast-enhanced MRI show large non-enhancing areas within the central gland consistent with treatment-related necrosis (*)
32.3 Prostate Tumor
A significant increase in the number of elderly people as a proportion of the total population has been occurred in the last half century with enormous demographic changes (Syrigos et al. 2005). These older men can anticipate a longer life with a consequent increase of cancer risk. It has been estimated that in the near future, 70 % of cancers will arise in people over 65. Prostate cancer is among the most common cancers in men, with only lung and colorectal cancer killing more men per year. Prostate carcinoma alone accounts for 28 % of incident cases in men, and around one in six men will be diagnosed with prostate cancer during his lifetime (Jemal et al. 2011).
Because of the shift in the age distribution of the population towards those over 65 years in whom 80 % of the tumors occur, the number of new cases and deaths per year will increase. Autopsy studies have shown a high number of histological prostate cancers, but the majority remain clinically silent and few become clinically manifest during lifetime (Fig. 32.7). Age is the greatest risk factor for prostate cancer, and the prevalence is about 30 % in men aged over 50 years, while small foci of adenocarcinoma can be detected by pathology in all men over 90 years. As a consequence of this increased life expectancy, there are many more elderly men with prostate cancer (Sangar et al. 2005). This phenomenon has led to an increased attention on prostate cancer detection and treatment in the aged, with specific emphasis on the problem of whether treatment should be different than younger patients.
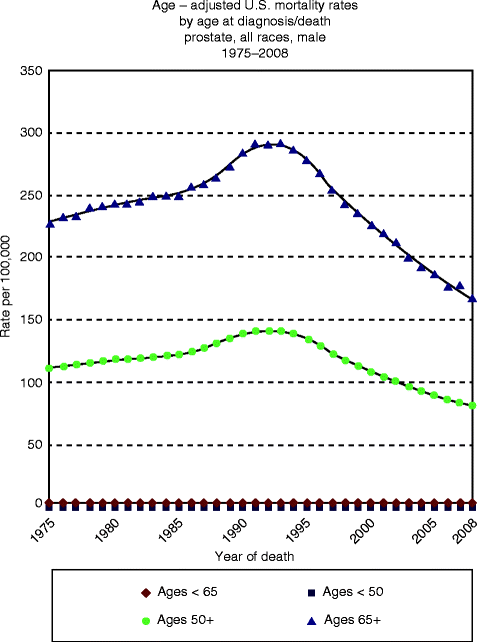

Fig. 32.7
Prostate cancer mortality estimated by age. The information is based on NCI’s SEER Cancer Statistics Review Prostate Section (http://seer.cancer.gov/csr/1975_2009_pops09/results_merged/sect_23_prostate.pdf)
Histological cancers found at systematic autopsy are described as latent, microscopic, incidental, or indolent, but there are no definite criteria to predict with accuracy which of these tumors will produce clinical disease (Stangelberger et al. 2008).
The increased incidence of prostate cancer with age is associated with an increase of common coexisting diseases such as arthritis, hypertension, cardiac disorders, and respiratory disorders (Repetto et al. 1997). Still clinically more important in these patients are the mental function disturbances and depressive disorders that complicate elderly patient care, restricting the diagnostic process and subsequent therapeutic decisions (Hall et al. 2005).
The prevalence of histological prostate cancers in men between 30 and 90 years does not differ significantly among different populations and is similar worldwide (Fig. 32.8), whereas the age-specific prevalence of clinical prostate cancer is different in men living in different countries (Mirza and Griebling 2011). These observations suggest that the lesions detected at autopsy are different biologically from clinical prostate cancer.
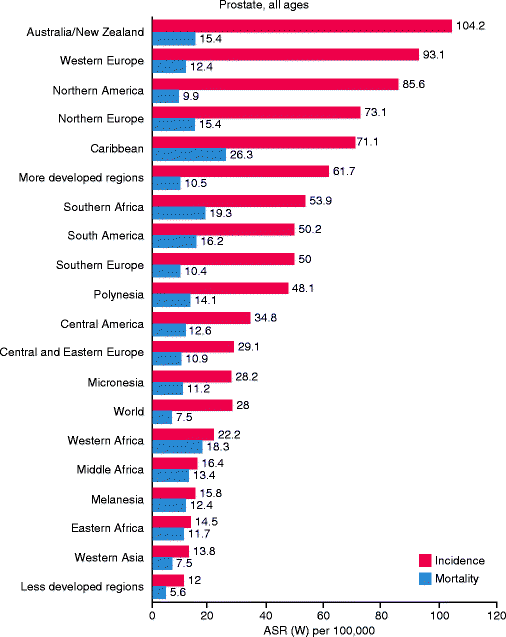

Fig. 32.8
International variation in age-standardized prostate cancer incidence and mortality rates (Source: GLOBOCAN 2008 (International Agency for Research on Cancer) (20.6.2011) (http://globocan.iarc.fr/factsheets/cancers/prostate.asp))
The natural history and the clinical course of the disease is challenging and unpredictable, ranging from a symptom-free, indolent condition without morbidity or negative effect on life span to an aggressive tumor that rapidly progresses to diffuse metastases and death (Concato et al. 2006). Between these two extremes lies a spectrum of clinical manifestations that influence the quality and the duration of life.
After 40 years of PSA identification and about 20 years of its use in clinical practice, there is still no general agreement on the usefulness of systematic prostate cancer identification, the utility of imaging procedures, or of the best treatment solutions in the elderly. PSA testing and the TRUS biopsy can reliably detect prostate cancer in an early preclinical phase with a high probability of effective therapeutic results.
Recently, the US Preventive Service Task Force (USPSTF) recommended against PSA-based screening. The task force pointed out that PSA is not cancer specific, prostate cancer aggressiveness varies widely, and the potential benefits of an early diagnosis of cancer and subsequent treatment that can be harmful are not always in the best interest of the patient (Brett and Ablin 2011).
In March 2009, the initial results of the two major screening trials were published, but they did not resolved the issue of PSA screening of prostate cancer. The US trial showed no mortality benefit from screening, but the European trial showed a small reduction of prostate cancer-related mortality.
Most authorities agree that there is no need for routine screening in men aged 75 years and older or those with medical general status that renders them unlikely to survive if the carcinoma becomes clinically symptomatic. Actually there is a general consensus that PSA should be tested in elderly men only in cases with clinically suspected cancer and a moderate PSA increase that should be correlated to age and volume of the gland (Heidenreich et al. 2011). Age-specific PSA reference ranges have been proposed to reduce the number of biopsies performed, because it is likely that many organ-confined tumors would be diagnosed, with questionable benefit for elderly, asymptomatic men.
Men with suspected prostate cancer present in primary care or urological evaluation with no clear symptoms of the disease. Commonly they have had a digital rectal examination and PSA evaluation. There have been published recommendation or guidelines from government organizations and scientific societies that emphasize the need for providing individualized information to men with suspected cancer, tailored to their own needs (Zhu et al. 2012). This information should be provided to the patient by the healthcare professionals, and all relevant diagnostic and therapeutic options should be discussed with the men and their partners or caregivers. The problem is still more imperative in men over 65 years of age and particularly in those that are 75 or 80 years of age.
Positive prostate biopsy is the only method for diagnosing prostate carcinoma, but a negative result does not exclude disease. The biopsy has the potential for causing harm: men with clinically insignificant prostate lesions may not benefit from knowing that they have a disease which is unlikely to cause symptoms or affect their life expectancy if they are over 75. The decision to perform a prostate biopsy should be discussed on the basis of digital rectal examination, PSA level, and comorbidities, including the risk factors and the results of previous negative biopsy results.
32.3.1 Imaging
Screening with PSA has led to increased detection of prostate cancer. The detected tumors are smaller, of lower grade and of lower stage. Therefore, validating imaging methods for prostate cancer detection, staging, and follow-up has become more challenging.
Recent studies on the use of imaging in elderly men with suspected or already diagnosed with prostate cancer showed that patients with lower risk tumors receive expensive imaging tests while a significant percentage of men at high risk for metastatic disease are not getting appropriate imaging tests. Imaging may be expensive, but many physicians order diagnostic imaging because patients demand the test or for fear of the legal consequences of missed metastases, even though the published guidelines or recommendations do not recommend them.
Ultrasound, MRI, and PET-CT are recommended for younger men with high-risk cancer, but recent results have shown that 36 % of men with low-risk and 49 % with intermediate-risk prostate cancer have undergone unnecessary imaging.
32.3.1.1 Ultrasound
Transrectal ultrasound (TRUS) of the prostate is the more diffuse imaging procedure used in the clinical practice after digital rectal examination and provides real-time imaging of the gland. Introduced by Watanabe et al. in 1967, it was considered for many years the best diagnostic method to detect prostate carcinoma. Lee et al. (1985) were the first to recognize that many patients had impalpable peripheral and central zone cancers that were detected only with biopsy. TRUS may demonstrate lesions in the peripheral zone of the prostate that are not palpable on rectal examination (Linden and Halpern 2007). However, the specificity is low and in fact, TRUS is mainly used to guide needle biopsy. This is an uncomfortable procedure, with some risk of infection and hemorrhage, and its value in older men is limited. The false-negative rate of biopsy is about 25 %, and considering the morbidity and discomfort, histological confirmation is useless since a negative biopsy does not exclude the presence of a prostate carcinoma.
On gray-scale ultrasound, prostate carcinoma is described as a hypoechoic lesion localized in the peripheral zone (Fig. 32.9) (Haffner et al. 2009). Hypoechoic peripheral and central zone nodules prove to be cancer only in one of three patients studied with multiple biopsies. Biopsies of hypoechoic areas show normal prostatic tissue or inflammatory areas or benign hyperplasia, indicating the lack of specificity. Peripheral and central zone cancers are isoechoic in 21 % of cases (Toi et al. 2007). Transition zone cancers which represent 20 % of all prostate tumors cannot be detected with ultrasound because of the heterogenicity of this specific prostate area (Fig. 32.10).
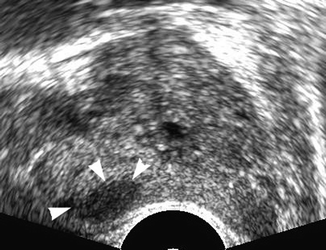
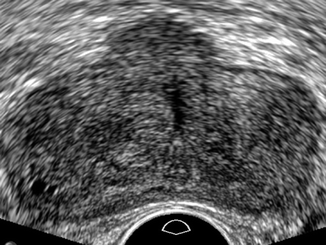

Fig. 32.9
Transrectal ultrasound of the prostate in transverse view. Hypoechoic lesion in the right side of the gland (arrow heads). TRUS-guided biopsy confirmed a prostate cancer of Gleason score 6 (3 + 3)

Fig. 32.10
Transrectal ultrasound of the prostate in transverse view. Normal ultrasound appearance of the gland. Subsequent MRI demonstrated prostate cancer in the transitional zone
Past studies have considered TRUS as a valid diagnostic procedure for screening early prostate cancer. At present, it is established that conventional TRUS is ineffective and is not useful in determining who has cancer and needs a biopsy.
The detection of a suspicious lesion on TRUS increases the likelihood of positive biopsy for cancer by a factor of 1.8 (7.8 % vs. 30.8 %), and the cancers are more likely to have a high Gleason score than cancers detected during randomly performed biopsies. In different studies of patients with elevated serum PSA values, TRUS was found to have a sensitivity of 41 % and specificity of 85 %.
Many studies have evaluated the clinical usefulness of color and power Doppler in detecting prostate cancer (Halpern et al. 2000), but the clinical efficacy remains controversial, specifically in men with intermediate level of PSA (less than 10 ng/ml). In prostate cancer, color Doppler can show an increased vascularization from hypoechoic nodules (Fig. 32.11) that is similar to benign hyperplastic nodules. Halpern et al. have demonstrated the absence of benefit of power Doppler over color Doppler in their prospective study. Evaluating vascularity, they reported a sensitivity of 27.3 and 44.4 % and a specificity of 83.9 and 70.5 % for power Doppler in TRUS (Halpern et al. 2000).
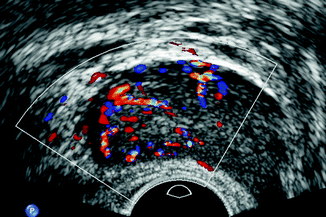

Fig. 32.11
Transrectal ultrasound of the prostate in transverse view. Color Doppler ultrasound shows focal hypervascularization in the right side of the gland, proven as prostate cancer
32.3.1.2 Contrast-Enhanced Ultrasound
Prostate carcinoma shows a variable biological aggressiveness that is associated with the grade of vascularity. Compared to normal tissue, cancer areas have a higher microvascular density that can be studied with ultrasound contrast agents.
Contrast-enhanced ultrasound with low mechanical index technology has been proposed to increase the diagnostic accuracy of TRUS and to target biopsies (Pelzer et al. 2005). Contrast-enhanced ultrasound may distinguish prostate cancer from areas of focal prostatitis on the basis of different vascular enhancement (Valentino et al. 2008) (Fig. 32.12). The identification of suspected areas in patients with elevated PSA levels allows guided biopsy, thus improving the probability of detecting a possible cancer. Cancer detection rate by targeted biopsy is better than standard sampling with a detection of 32.6 % versus 17.9 %. Contrast-enhanced ultrasound-targeted biopsies were 1.5 times more likely to find tumor than systematic random biopsy (Pallwein et al. 2008). The sensitivity of contrast-enhanced ultrasound-guided biopsy was 68 %, more than the 39 % of gray-scale and 41 % of color Doppler (Zhu et al. 2010). A significant benefit of contrast-enhanced ultrasound-targeted biopsy has been reported by Mitterberger et al. (2010). Target biopsy of hypervascular areas in the peripheral zone compared with random biopsy performed in 1,776 patients detected cancer in 31 % of patients (559), including 476 (27 %) patients with contrast-enhanced ultrasound and 410 (23 %) with random biopsy (p < 0.001). The detection rate of contrast-enhanced ultrasound-targeted biopsy cores was 10.8 %, significantly more accurate than for random biopsy which was only 5.1 % (p < 0.001). Among patients with cancer, the lesion was identified using contrast-enhanced ultrasound alone in 27 % of cases compared to random biopsy that detected only 15 % of tumors (p < 0.001). The drawback of the method was that anteriorly located tumors were not studied, with radical prostatectomy as the reference standard.
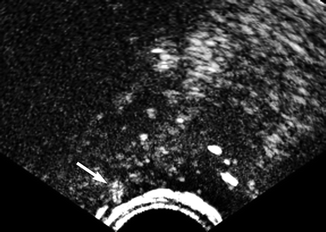

Fig. 32.12
Transrectal contrast-enhanced ultrasound of the prostate. Area with increased signal in arterial phase at the right side of the prostate (arrow). The suspicion of cancer because of early enhancement was confirmed by the biopsy
32.3.1.3 Elastography
Recently TRUS elastography has been introduced in clinical practice with some promising results. The technology used to evaluate tissue stiffness varies among devices with different modalities for producing the mechanical compression and different algorithm to calculate the stress share of tissue (Fig. 32.13).
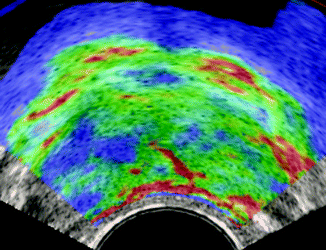

Fig. 32.13
Sonoelastography of prostate cancer: asymmetric signal of the gland, with a large area of increased stiffness (blue area) in the right side
In a recent paper, Walz et al. (2011) have shown that elastography alone is not reliable for the identification of prostate cancer areas. Combining elastography and randomized 12-core biopsy, they have found some promising results in correctly identifying preferred tumoral areas to biopsy.
In another study (Pallwein et al. 2008), comparing the results of TRUS elastography and MRI imaging to pathological findings, the diagnostic sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were interesting and promising. The reported results, with a specificity of elastography higher than that of MRI, appear excessively optimistic and need confirmation by more extensive use in the clinic.
In fact, the clinical role of TRUS elastography has not been defined, or even whether it can be used in association with other diagnostic methods to increase the detection rate or to reduce biopsies. It needs validation with more extensive research and comparison with radical prostatectomy results.
32.3.1.4 Magnetic Resonance Imaging
MRI using various anatomical and functional sequences has been shown to be effective for preoperative detection and local staging of prostate cancer in many studies. In the initial clinical application of MRI, the major benefits regarded the local staging before treatment, with lower sensitivity in the detection of intraprostatic focal cancers.
Recent technology with the use of multiparametric MRI has shown accurate tumor detection with high sensitivity and specificity. The MRI sequences commonly used are T2-weighted, diffusion-weighted, and dynamic contrast-enhanced MRI. These sequences allow tumor detection not only in the peripheral zone of the gland but in the central gland as well, where a significant overlap between tumors and benign hyperplasia is frequently observed. Prostate spectroscopy imaging is less frequently employed because of technical problems and the time required for a complete sampling of the metabolic activity of the gland.
T2-weighted MRI is the mainstay of morphological prostate MRI, providing the best depiction of the prostate’s zonal anatomy and capsule. Prostate cancer typically manifests as focal not well-defined, low-signal-intensity area in the peripheral zone (Fig. 32.14). However, various conditions such as prostatitis, hemorrhage, atrophy, and posttreatment changes can mimic cancer (Qayyum et al. 2004; Oto et al. 2010). Tumors localized to the transition zone or central gland are more challenging to detect since the signal intensity characteristics of the normal and hypertrophic transition zone usually overlap with tumor (Turkbey et al. 2009). T2-weighted MRI performed at 1.5 T with endorectal and phase-array coils has sensitivity and specificity of 35–82 % or detection and 75–99 % for local staging (Sala et al. 2006; Futterer et al. 2007) (Fig. 32.15). Reported sensitivity and specificity at 1.5 T with phase-array coil only are lower: 41–62 and 69 %, respectively (Yoshizako et al. 2008; Shimizu et al. 2009




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree